Reaction Rates - Madison Public Schools
advertisement

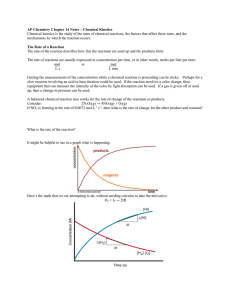
Reaction Energy and Reaction Kinetics Reaction Rate Objectives 1. Define the rate of reaction and describe how it can be determined. 2. List and explain factors that affect the rate of reaction. 3. Define a catalyst and describe how it can affect the rate of reaction. Reaction Rate Reaction rate – change in concentration of reactants per unit time as a reaction occurs. Chemical kinetics – study of reaction rates and reaction mechanisms. Rate-Influencing Factors Nature of the Reactants H2(g) + Cl2(g) 2 HCl(g) 3 H2(g) + N2(g) 2 NH3(g) (fast) (slow) 4 Na(s) + O2(g) 2 Na2O(s) 4 Fe(s) + 3 O2(g) 2 Fe2O3(s) (fast) (slow) Rate-Influencing Factors Surface Area • Heterogeneous reactions – reactions with reactants in two different phases • Heterogeneous reactions depend on contact between the two phases. • Increases in surface area result in the increase in the rate of heterogeneous reactions. Rate-Influencing Factors Temperature An increase in temperature causes: • An increase in collision energy • An increase in collision frequency Both result in increased reaction rates • In general, a 10oC increase in temperature causes the reaction rate to double. Rate-Influencing factors Concentration • Increasing the concentration of the reactants may cause an increase in the rate of reaction for homogeneous reactions. Rate-Influencing Factors Presence of a Catalyst • Catalysts lower the activation energy for a reaction and, thus, increase the reaction rate. • Homogeneous vs. Heterogeneous catalysts – same or different phase as reactants. Rate Laws for Reactions Objectives: 1. Explain the rate law for a chemical reaction. 2. Determine the rate law for a particular reaction given appropriate kinetics data. 3. Discuss the relationship between the rate law and the reaction mechanism. Rate Law Rate Law – an equation that relates reaction rate and concentration of reactants R = k[A]n[B]m… R = reaction rate k = rate constant that depends on temperature n,m = must be determined experimentally Rate Laws for Reactions Example: 2 H2(g) + 2 NO(g) N2(g) +2 H2O(g) • Doubling the amount of H2 , while keeping the amount of NO constant, causes the reaction rate to double. So… R [H2] • When the amount of NO is doubled, while keeping the amount of H2 constant, causes the reaction rate to increase fourfold. So… R [NO]2 Overall rate law: R = k[H2][NO]2 Rate Laws for Reactions For reactions that occur in a single step: A + B 2C R = k[A][B] For the reverse reaction…. 2C A + B R = k[C]2 Rate Laws for Reactions For multi-step reactions, the rate law depends on the rate limiting step. Example: NO2(g) + CO(g) NO(g) + CO2(g) Step 1: NO2 + NO2 NO3 + NO Step 2: NO3 + CO NO2 + CO2 Rate Law: R = k[NO2]2 (slow) (fast) Sample Problems 1. The rate law of a reaction is found to be R=k[X]3. By what factor does the rate increase if the concentration of X is tripled? The rate will increase by a factor of 27. 2. The rate of reaction involving two reactants, X and Z, is found to double when the concentration of X is doubled and to quadruple when the concentration of Z is doubled. Write the rate law for this reaction. R = k[X][Z]2 3. The rate law for a single-step reaction that forms one product, C, is R = k[A][B]2. Write the balanced reaction of A and B to form C. A + 2B C Sample Problem • A particular reaction is found to have the following rate law: R = k[A][B]2 How is the rate affected by each of the following changes? a. The initial concentration of A is cut in half. b. The initial concentration of B is tripled. c. The concentration of A is doubled, but the concentration of B is cut in half. d. A catalyst is added. Sample Problem A chemical reaction is expressed by the balanced chemical equation, A + 2B C. Using the data below, answer the following: a. Determine the rate law for the reaction b. Calculate the value of the specific rate constant. c. If the initial concentrations of both A and B are 0.30 M, at what initial rate is C formed? Experiment # Initial [A] Initial [B] Initial rate of formation of C 1 0.20 M 0.20 M 2.0x10-4M/min 2 0.20 M 0.40 M 8.0x10-4M/min 3 O.40 M 0.40 M 1.6x10-3M/min