AP Chemistry MC - Rancho High School
advertisement

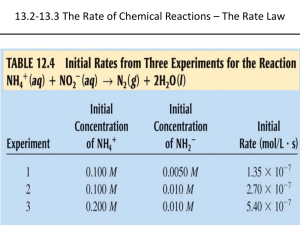
Chemistry MC For the reaction 2A + 3B + C = Products, the rate equation is: a) R = k[A][B][C] b) R = k[A]2[B]3[C] c) R = k[B]3[C] d) Insufficient information to fix rate equation Consider 2A + 3B = D When Designing The Experiment If You Would Like To Determine The Rate Order With Respect To A You Notice That When You Double The Concentration The Rate Doesn’t Change. What Can You Conclude From That a) It Is Zero Order For A b) It Is First Order For A c) It Is Second Order For A d) A Doesn’t Have Impact So It Is Not Part Of The Rate Law 1. Which graph represents a plot of average rate on the vertical axis versus the average concentration of A squared on the horizontal axis for a second order reaction. 2. Which graph represents a plot of the natural logarithm of the concentration of A on the vertical axis versus the time on the horizontal axis for a first order reaction The following reaction is found to be first order in H2 (g) and second order in NO (g). The rate law for this reaction is: 2NO (g) + 2H2 = N2 (g) + 2H2O (g) A)Rate = k[NO]2[H2]2/[N2][H2O]2 B) Rate = k[NO][H2]2 C) Rate = k[NO]2[H2]2 D) Rate = k[NO]2[H2] E) Rate = k[N2][H2O]2/[NO]2[H2] Which statement describes characteristics of an endothermic reaction? A. B. C. D. The sign of H is positive, and the products have less potential energy than the reactants. The sign of H is positive, and the products have more potential energy than the reactants. The sign of H is negative, and the products have less potential energy than the reactants. The sign of H is negative, and the products have more potential energy than the reactants. For irreversible chemical reactions the rate will be affected by changes in all of these factors except: A) temperature B) Concentration of reactants C) presence of a catalyst D) concentration of products E) surface area of solid reactant. For the following reaction: NO2(g) + CO(g) → NO(g) + CO2(g), the rate law is: Rate = k[NO2]2. If a small amount of gaseous carbon monoxide (CO) is added to a reaction mixture that was 0.10 molar in NO2 and 0.20 molar in CO, which of the following statements is true? a) Both k and the reaction rate remain the same. b) Both k and the reaction rate increase. c) Both k and the reaction rate decrease. d) Only k increases, the reaction rate remains the same. e) Only the reaction rate increases; k remains the same. Bozeman and HW Quiz 1. In Bozeman’s video on the reaction path he talks about the temperature dependence. We know that reaction rate influences T but is k dependent on T. Explain. 2. Describe the experiment and scientist Bozeman mentioned in the video regarding Multistep reactions. What was it about? Write whatever you can remember about it? 3. Chemical reaction happens in a test tube you are holding and as this happens your hand feels hot. Sketch the E diagram for this reaction. 1. Describe the experiment and scientist Bozeman mentioned in the video regarding Multistep reactions. What was it about? Write whatever you can remember about it? 2. Chemical reaction happens in a test tube you are holding and as this happens your hand feels hot. Sketch the E diagram for this reaction. 3. Define transition state and draw a diagram showing it. Bozeman and HW Quiz 1. 2. Describe the experiment and scientist Bozeman mentioned in the video regarding Multistep reactions. What was it about? Write whatever you can remember about it? Chemical reaction happens in a test tube you are holding and as this happens your hand feels cold. Sketch the E diagram for this reaction. The specific rate constant, k, for radioactive beryllium–11 is 0.049 s–1. What mass of a 0.500 mg sample of beryllium–11 remains after 28 seconds? a) b) c) d) e) 0.250 mg 0.125 mg 0.0625 mg 0.375 mg 0.500 mg The slow rate of a particular chemical reaction might be attributed to which of the following? a) a low activation energy b) a high activation energy c) the presence of a catalyst d) the temperature is high e) the concentration of the reactants are high The steps below represent a proposed mechanism for the catalyzed oxidation of CO by O3. Step 1: NO2(g) + CO(g) → NO(g) + CO2(g) Step 2: NO(g) + O3(g) → NO2(g) + O2(g) What are the overall products of the catalyzed reaction? a) CO2 and O2 b) NO and CO2 c) NO2 and O2 d) NO and O2 e) NO2 and CO2 Which energy diagram represents a highly exothermic reaction that has a small activation energy? (Assume that all curves are plotted on the same scale.) Which procedure will increases the solubility of KCl in water? a) stirring the solute and solvent mixture b) increasing the surface area of the solute c) raising the temperature of the solvent d) increasing the pressure on the surface of the solvent The rate of a reaction with just two reactants is observed to double when the concentration of one reactant is doubled and the second reactant is held constant. The rate is also observed to increase by a factor of nine when the concentration of the second reactant is tripled, holding the concentration of the first reactant constant. What is the overall order for this reaction? (A) 2 (C) 5 (B) 3 (D) 6