notes
advertisement

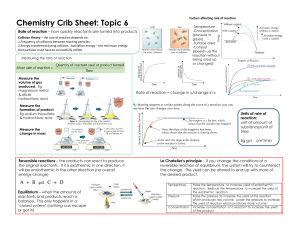
Jon Gillespie Evan Rosenfeld Unit 15 – Kinetics: Rates Of Chemical Reactions: Rate= Change of some quantity / time For example, Δ distance / time = velocity Rate of reaction = -Δ[N2O] / Δ time Reaction Conditions And Rate: k = Ae(-Ea)/(RT) Where A is the percent of properly aligned collisions, Ea is activation energy, T is temperature, and R is 8.314 JK-1 mol- Concentration of Reactants this is because the rate is proportional to the concentration of reactants, so as the reaction proceeds and the reactants are used up, rate decreases Temperature T is proportional to KE which is proportional to velocity (squared). As T increases, molecules have more frequent, more forceful collisions, speeding up the reaction rate Catalysts Catalysts provide an alternative path for reactions, one with much lower activation energy. Therefore, catalysts speed up the rate of reaction. However, they speed up the forward and reverse reactions equally, so there is no change in the k. A Microscopic View of Reactions: k = Ae(-Ea)/(RT) Collision theory states two conditions that must be met for a collision to occur The molecules must collide properly i.e. have the correct orientation They must collide with sufficient energy i.e. Ea must be met Writing Rate Equations: Rate of a Reaction = K*[A]a[B]b Where A and B are reactants of the reaction And a and b are determined by analysis of experimental data The Relationship Between Concentration and Time: When concentration versus time is graphed k or the rate constant is equal to the slope Linear slopes only result from certain variables that depend upon the order of the reaction Mechanisms: Reactions are composed of various elementary steps The slowest elementary step is the rate determining step Rate equations for elementary steps are based off stoichiometry If the rate determining step has an intermediate, you must substitute to get it in terms of the reactants