ITC (Isothermal Titration Calorimetry)
advertisement

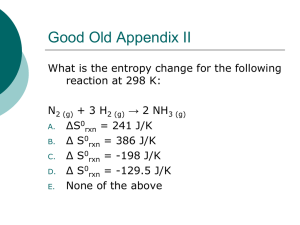
A single experiment sufficient to obtain all of the thermodynamic components Stoichiometry of the interaction (n) Association constant (Ka) & Dissociation constant (Kd) Enthalpy (ΔHb) Free energy (ΔGb) Entropy (ΔSb) Heat capacity of binding (ΔCp) A system is defined as the matter within a defined region of space (i.e., reactants, products, solvent) The surroundings is the matter in the rest of the universe The total kinetic energy due to the motion of molecules (translational, rotational, vibrational) + the total potential energy associated with the vibrational and electric energy of atoms within molecules or crystals. ΔU= W + Q ΔU= W + Q QP = ΔU – W QP = ΔU – P(V2-V1) QP = ΔU – P(ΔV) QP = ΔH The enthalpy is the heat absorbed or emitted by a system at constant pressure Exothermic reaction ΔH is negative Emits heat Endothermic reaction ΔH is positive Absorbs heat Reaction continues Temperature change occurs Power supply is given to maintain a constant temperature difference between the reaction cell and the reference cell Power supply is measured Exothermic reaction: Emit heat Negative peak on ITV Endothermic reaction: Absorb heat Positive peak on ITC Quantitative technique that can directly measure: the binding affinity (Ka) enthalpy changes (ΔH) binding stoichiometry (n) of the interaction between two or more molecules in solution Gibbs energy changes (ΔG), and entropy changes (ΔS), can be determined using the relationship: ΔG = -RTlnKa = ΔH-TΔS (where R is the gas constant and T is the absolute temperature). As promised… The Energy is conserved The total energy of a system and its surroundings is constant In any physical or chemical change, the total amount of energy in the universe remains constant, although the form of the energy may change. ΔU= W + Q ΔE represents the change in the energy Q the heat absorbed by the system W the work done on the system QP = ΔU – W QP = ΔU – P(V2-V1) QP = ΔU – P(ΔV) QP = ΔH The enthalpy is the heat absorbed or emitted by a system at constant pressure The total entropy of a system and its surroundings always increases for a spontaneous process ΔStotal = Δ Ssystem + Δ Ssurroundings Δ Ssurroundings = - Δ Hsystem/T Δ Stotal = Δ Ssystem - Δ Hsystem/T -T Δ Stotal = Δ Hsystem - T Δ Ssystem Δ G = Δ Hsystem - T Δ Ssystem ΔG<0 spontaneous change ΔG=0 equilibrium For a reaction to be spontaneous, the entropy of the universe, ΔStotal, must increase: Δ Ssystem > Δ Hsystem/T or Δ G = Δ Hsystem – T Δ Ssystem < 0 The free energy must be negative for a reaction to be spontaneous! Reactant Product Association constant: ΔG = –RT lnKeq Keq = 10–ΔG/1.36 PL G G RT ln PL 0 at steady state, at which ΔG=0 PL G RT ln PL 0 1 G RT lnK eq RT ln Kd H 0 1 S 0 lnK d R R T 0 RT lnK d ADVANTAGES Immobilization or labeling 필요 없다. Kd, ΔH can be measured Can be applied to different reaction temperature and pH DISADVANTAGE Enormous amounts of binding partner ◊ Only medium affinity ◊ Limitation for membrane proteins ◊ High price ◊