Document
advertisement

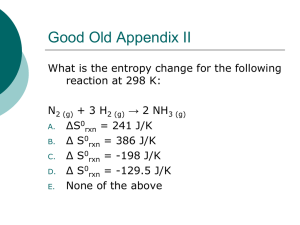
ENERGY The ability to do work or produce heat. It exists in two basic forms, potential energy and kinetic energy: Potential: energy due to the composition or position of an object Kinetic: energy of motion Chemical potential energy is the energy stored in a substance because of its composition. ENERGY FLOW Exothermic: when heat flows from the object to its surroundings; heat is released Endothermic: when heat flows from the surroundings to the object; heat is absorbed Is making ice cream exothermic or endothermic? LAW OF CONSERVATION OF ENERGY In any chemical reaction or physical process, energy can be converted from one form to another, but is neither created nor destroyed. HEAT (Q) Energy that is in the process of flowing from a warmer object to a cooler object. The amount of heat required to raise one gram of water by one degree Celsius is defined as a calorie (cal). 1 Calorie = 1 kcal = 1000 cal The SI unit of heat and energy is the joule (J). CONVERTING ENERGY UNITS 1 J = 0.2390 cal 1 cal = 4.184 J A granola bar contains 142 Calories. Convert this to joules. SPECIFIC HEAT The amount of heat required to raise the temperature of one gram of that substance by one degree Celsius. q = m · c · ΔT q = the heat absorbed or released m = the mass of the sample in g c = the specific heat of the substance ΔT = the change in temperature CALCULATIONS WITH SPECIFIC HEAT The temperature of a sample of iron with a mass of 10.0g changed from 50.4˚C to 25.0˚C with the release of 114J heat. What is the specific heat of iron? If the temperature of 34.4g of ethanol increases from 25.0˚C to 78.8˚C, how much heat has been absorbed by the ethanol? The specific heat of ethanol is 2.44J/(g·˚C) MEASURING HEAT A calorimeter is an insulated device used for measuring the amount of heat absorbed or released during a chemical or physical process. BOMB CALORIMETER THERMOCHEMISTRY The study of heat changes that accompanies chemical reactions and phase changes. System: the specific part of the universe that is being studied (chemical reaction) Surroundings: everything else Universe = system + surroundings ENTHALPY (H) The heat content of a system at constant pressure Enthalpy of reaction(ΔHrxn) is the change in enthalpy for a reaction ΔHrxn= Hfinal – Hinitial ΔHrxn= Hproducts - Hreactants If ΔH is positive, the reaction is endothermic If ΔH is negative, the reaction is exothermic THINK-PAIR-SHARE Compare energy changes in chemical reactions to profits and losses in a business. Each month the business has receipts (positive dollar amounts) and expenses (negative dollar amounts). If the total receipts exceed expenditures, a positive dollar amount or profit occurs. If expenditures are greater than receipts, money is lost and a negative dollar amount results. PRACTICE PROBLEM A 75.0g sample of a metal is placed in boiling water until its temperature is 100.0˚C. A calorimeter contains 100.00g of water at a temperature of 24.4˚C. The metal sample is removed from the boiling water and immediately placed in the water in the calorimeter. The final temperature of the metal and water in the calorimeter is 34.9˚C. Assuming that the calorimeter provides perfect insulation, what is the specific heat of the metal? THERMOCHEMICAL EQUATION A balanced equation that includes the physical states and energy change. Example: 4Fe(s) + 3O2(g) → 2Fe2O3(s) ΔH= -1625 kJ ENTHALPY (HEAT) OF COMBUSTION (ΔHCOMB) The enthalpy change for the complete burning of one mole of a substance Example: C6H12O6(s) + 6O2(g) → 6CO2(g) + 6H2O(l) ΔHcomb=-2808kJ CHANGES OF STATE Molar enthalpy (heat) of vaporization (ΔHvap) H2O(l) → H2O(g) ΔHvap= 40.7kJ H2O(g) → H2O(l) ΔHcond= -40.7kJ Molar enthalpy (heat) of fusion (ΔHfus) H2O(s) → H2O(l) ΔHfus= 6.01kJ H2O(l) → H2O(s) ΔHsolid= -6.01kJ What would be the molar enthalpy of sublimation? PRACTICE PROBLEMS Calculate the heat required to melt 25.7 g of solid methanol at its melting point. ΔHfus=3.22 kJ/mol How much heat is evolved when 275 g of ammonia gas condenses to a liquid at its boiling point? ΔHvap=23.3 kJ/mol What mass of methane must be burned in order to liberate 12,880 kJ of heat? ΔHcomb=-891 kJ/mol PROBLEM-SOLVING LAB Pg. 531 Make, Explain and Use the Graph Brady and Antonio Rule!!!!!!! HESS’S LAW Read section on p. 534 and 535 Add the following equations: A+B→C C+D→E+B EXAMPLE Ex) 2S(s) + 3O2(g) → 2SO3(g) ΔH= ? S(s) + O2(g) → SO2(g) ΔH= -297kJ 2SO3(g) → 2SO2(g) + O2(g) ΔH= 198kJ EXAMPLE Use reactions a and b to determine ∆H for the following reaction. 2CO + 2NO → 2CO2 + N2 ∆H = ? A) 2CO + O2 → 2CO2 ∆H = -566.0 kJ B) N2 + O2 → 2NO ∆H = 180.6 kJ 4Al + 3MnO2 → 2Al2O3 + 3Mn ∆H = ? 4Al + 3O2 → 2Al2O3 ∆H = -3352 kJ Mn + O2 → MnO2 ∆H = -521 kJ SPONTANEOUS PROCESS A spontaneous process is a physical or chemical change that occurs with no outside intervention. Ex. Iron forming rust, combustion of methane, melting of ice cream A non-spontaneous change is a change that occurs only when driven e.g. forcing electric current through a metal block to heat it What makes a reaction spontaneous? WHAT DETERMINES IF A REACTION IS SPONTANEOUS? Entropy: Measure of the disorder or randomness of the particles that make up a system. Spontaneous processes always proceed in such a way that the entropy of the universe increases. (ΔS) ΔSuniverse= ΔSsystem + ΔSsurroundings Larger entropy value, larger degree of randomness. Law of Disorder (second law of thermodynamics): Spontaneous processes always proceed in such a way that the entropy of the universe increases. LAW OF DISORDER (SECOND LAW OF THERMODYNAMICS) Law of disorder: Spontaneous processes always proceed in such a way that the entropy of the universe increases. Entropy gas > entropy liquid > entropy solid Dissolving of a gas in a solvent always results in a decrease in entropy An increase in temperature results in an increase in entropy. Entropy increases when the number of product particles is greater than the number of reactant particles. 2SO3 (g) 2SO2 (g) + O2 (g) ΔSsystem > 0 ENTROPY, THE UNIVERSE AND FREE ENERGY For any spontaneous reaction ΔSuniverse > 0 ΔSuniverse is positive when, 1. the reaction is exothermic 2. The entropy of the system increases, so ΔSsystem is positive Gibbs Free Energy (G) or Free energy: -the energy that is available to do work. ΔGsystem= ΔHsystem -TΔSsystem Type of reaction or process ΔGsystem ΔSuniverse Spontaneous Negative Positive Nonspontaneous Positive negative How ΔHsystem and ΔSsystem Affect Reaction Spontaneity -ΔHsystem +ΔHsystem +ΔSsystem Always spontaneous Spontaneity depends upon temperature -ΔSsystem Spontaneity depends upon temperature Never spontaneous PRACTICE PROBLEM For a process, ΔHsystem= 145 kJ and ΔSsystem=322 J/K. Is the process spontaneous at 382 K?