PowerPoint 5.1 - The First Law and Enthalpy
advertisement

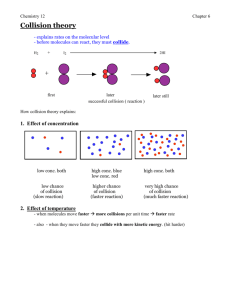
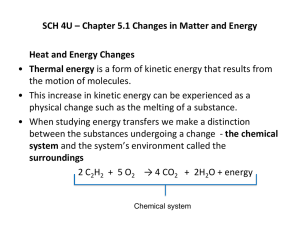
Catalyst Complete the exam reflection sheet using the data from the exam. Remainder of the Year Monday Tuesday Wednesday Thursday Friday 15th 16th Unit 5 Unit 5 NO SCHOOL! NO SCHOOL! 19th 20th 21st Unit 5 Unit 5 Unit 5 26th 27th 28th 29th 30th Unit 5 Unit 5 Unit 5 Unit 5 Unit 5 3rd Review and Quest 4th Review and Quest 5th 6th 7th Review Review Review 10th MOCK AP EXAM 11th MOCK AP EXAM 12th Review Exam 13th 14th Last day of classes Whoosh Bottle Justify – TPS Why is heat generated during this reaction: CH3CH2OH + 3O2 3H2O + 2CO2 Lecture 5.1 – 1st Law of Thermodynamics and Enthalpy Today’s Learning Targets LT 5.1 – I can discuss the energy associated with a system using the ideas of work and heat. LT 5.2 – I can compare and contrast the idea of system and surrounding when examining a given substance LT 5.3 – I can discuss the First Law of Thermodynamics, how it relates to the quantitative description of the energy of a system, and how it describes endothermic/exothermic reactions. LT 5.4 – I can define what a state function is and apply this definition to thermodynamic problems LT 5.5 – I can relate the 1st law of thermodynamics to the concept of enthalpy and I can apply this concept to chemical reaction and endothermic/exothermic. What is Energy? Energy is the capacity of a system to do work and/or transfer heat All processes require some form of energy Energy is classified as being either: Kinetic Energy – The energy of motion Potential Energy – The energy associated based on an objects position. Two Energy Forms: Work and Heat Work is the energy used to cause an object to move against a force WorkForceDistance Heat is the energy used to cause the temperature of an object to increase ALWAYS transfers from one object to another object Two Types of Energy: Kinetic and Potential There are both energy of motion and energy of location Kinetic Energy is energy of motion and is calculated by: 1 2 KE mv 2 Potential Energy is energy based on location/position of an object. Bonds have energy based on composition. All energy is measured in Joules and can be converted to calories: 1 calorie = 4.184 J 1 Cal = 1 kcal = 1000 calories System and Surrounding When studying the movement of energy we need to define system and surroundings System – The portion that we will be studying Surrounding – Everything else that is not be studied Types of Systems Open System – Matter and Heat can be exchanged Closed System – Heat can be exchanged, but matter cannot Isolated System – No exchange of heat and matter 1st Law of Thermodynamics 1st Law of Thermodynamics states that energy cannot be created or destroyed, therefore it must be conserved. Used to analyze energy changes in chemical systems An increase in energy of system leads to an equal decrease of surroundings, and vice versa Internal Energy (E) Internal Energy is the sum of all kinetic and potential energies of the components of a system. We are concerned with the change in internal energy (ΔE) E E final Einitial Esurroundings Esystem Euniverse 0 Efinal > Einitial indicates system gained from surroundings and ΔE is positive Efinal < Einitial indicates system lose to the surroundings and ΔE is negative Heat, Work, and the 1st Law ΔE is exchanged in the form of either heat or work If you remove heat (q < 0) and/or work is done by the system (w < 0) , then ΔE is negative If you add heat (q > 0) and/or work is done by the system (w > 0), then ΔE is positive Sign Conventions When the system increases, then values are positive When the system loses, the values are negative Heat, Work, and the 1st Law We, therefore, can describe the internal energy by: ΔE = q + w You must decide on signs for q and w! Class Example Gases A (g) and B (g) are confined in a cylinder with a moveable piston. These gases react by the following reaction: A (g) + B (g) C (s) As the reaction occurs, the system loses 1150 J of heat to the surroundings. The piston moves downward as the gases react to form a solid. As the volume of the gas decreases under the constant pressure of the atmosphere, the surroundings do 480 J of work on the system. What is the change in internal energy? Table Talk Calculate the change in the internal energy for a process in which a system absorbs 140 J of heat from the surroundings and does 85 J of work on the surroundings. Relay Races Two points back on your exam to the winning team! Relay Race Questions What does the 1st Law of Thermodynamics State You add heat to a glass of water. Define system and surrounding in this scenario. Work is done by the system. Should w be positive or negative? Calculate ΔE when q = 0.763 kJ and w = -840 J. Is it endothermic or exothermic? Calculate ΔE when a system releases 66.1 kJ of heat to its surroundings while the surroundings do 44.0 kJ of work on the system Justify – TPS Using the idea of heat, system and surrounding, why was it beneficial for the three men to cuddle? Endothermic/Exothermic When heat is transferred to a system from the surroundings, it is endothermic Flow of Heat When heat is transferred from a system to the surroundings, it is exothermic Flow of Heat Road Trip! 5280 ft 524 ft For each route, what is the change in altitude? State Functions State functions are values that depend only on the present state of the system, not on the path the system took to reach that state ΔE is a state function, but q and w are not This means that if q is increased, then w decreases by the same amount Think of q and w being the different paths taken Other state functions are T, P, and V Enthalpy We combine three state functions (P, V, and E) to create the state function of enthalpy that is used to describe the flow of energy into/out of a system H = E + PV The change in enthalpy equals the heat gained or lost at constant pressure See derivation on the board ΔH = qP Meanings of Enthalpy Values +ΔH = system gained energy; endothermic process -ΔH = system lost energy; exothermic process Class Example Indicate the sign of ΔH in these processes carried out under atmospheric pressure and indicate whether it is exothermic or endothermic: An ice cube melts Table Talk Indicate the sign of ΔH in these processes carried out under atmospheric pressure and indicate whether it is exothermic or endothermic: 1 g of butane is combusted in sufficient oxygen to give complete combustion to CO2, H2O, and a release of energy 1st Law Advertisement You are working for an advertisement company and some scientists have asked you to create an advertisement for the first law Your clients have required that you include the following terms/ideas: Energy Work Heat System/Surrounding Internal Energy Enthalpy State Functions Exit Ticket Closing Time Read 5.1 – 5.3 Do book problems: 5.3, 5.4, 5.6, 5.9, 5.15, 5.25, 5.26, 5.27, and 5.28