Lab 5: - WordPress.com
advertisement

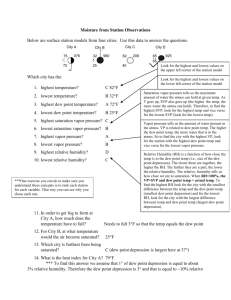
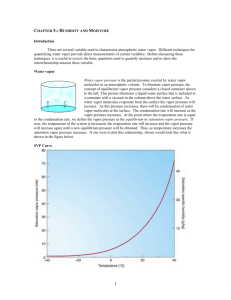
Lab 5: Atmospheric Moisture Relative Humidity • Sling Psychrometers: measures Relative Humidity • Dry bulb temp • Web bulb temp DB – WB = Wet bulb depression DB vs. WB: Big difference = dry air Small difference = moist air Relative Humidity • Swing for ~ 60 seconds • If you have a fraction, change to a whole number • Record DB & WB temps • Individuals or groups • 4 locations: • Inside • Outside Measuring Relative Humidity Dry Bulb Temp Saturation Mixing Ratio (SMR) Based on DB temp; TABLE 2 Wet Bulb Temp Wet-Bulb Depression Relative Humidity (RH) Mixing Ratio (MR) DB – WB Based on DB temp & WBD; TABLE 4 SMR * RH (RH is a %!) Atmospheric Moisture: Latent Heat • • • • • Water comes in three phases: – Solid – Liquid – Vapor • Unique to this atmospheric component *** Latent heat transfer: process of water changing phase – Energy is consumed or released – Sensible heat: heat we can feel & measure – Latent heat: energy in the form of heat Sensible heat: potential energy in the form of thermal energy or heat Latent heat: amount of energy in the form of heat released or absorbed by a chemical substance during a change of state. Kelvin is the most reliable measurement of temperature because zero kelvin represents the temperature at which all molecular motion stops. • • • Heat: average kinetic energy of a given amount of liquid Condensation = warming process Evaporation = cooling process Atmospheric Moisture: Latent Heat Phase change Energy Examples Ice → (liquid) water Consumed Ice cubes melting in a glass; melting of falling snowflake Water → water vapor Consumed Clothes drying; evaporation off a water surface Ice → water vapor Consumed Solid air freshner; sublimation of snow/ice Water vapor → water Released Dew; condensation on a cold can of pop; contrail Water vapor → ice Released Frost on grass or on a window Water → ice Released Freezing ice cubes • Amount of water vapor in the air depends on amount of energy available to change liquid → gas. – As temperature increases, the liquid water molecules start moving faster…it’s more likely to evaporate • Energy NOT used to boil the water is used to change the state of the water from liquid to gas • Two ways to measure atmospheric moisture: 1. Vapor pressure – Water vapor molecules exert pressure proportional to their concentration in the atmosphere Maximum is called saturation vapor pressure • SVP increases with temperature 2. Mixing Ratio – – Mass of water vapor in the air – Ratio of water vapor mass to the mass of dry air • Units of grams of water vapor per kilogram of dry air – saturation mixing ratio: air is saturated • Depends on temperature (↑ with temperature) • Represents max weight of water vapor/kilogram of dry air Relative Humidity: measures how close the air sample is to saturation • It is a ratio of actual water vapor in the air to the saturation level at a given temperature. • The greater the difference between air temperature & the dew point, the lower the RH (dry air) • RH is temperature dependant Winter? Warming up already dry air will DECREASE Relative Humidity! •Does winter indicate air that would be dry or moist? In the winter, air is generally rather dry. Cold air has less capacity to hold water vapor than warm air. •Heat in your home – becomes dry Summer? • In the summer, air is generally moist. Warm air can hold greater amounts of water vapor than cold air. • Cooling already moist air will INCREASE Relative Humidity! Dew Point • Dew point: temperature to which air must be cooled to reach saturation (RH = 100%) – Generally seen as dew in the morning on the grass. Dew Point 1. Find MR of 17 2. Create a STRAIGHT line to the dew point line 3. Create another STRAIGHT line down to the temperature 4. Read the temperature. A 30° air sample would have to cool to ~ 22° to reach saturation (100% humidity)