Introduction only
advertisement

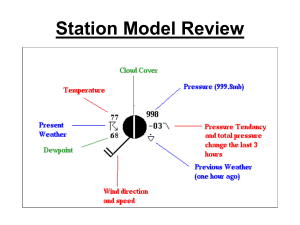
The Comparison Between Measuring Dew Point Temperature with a Tin Can Filled with Ice and a Sling Psychrometer, and the Reasoning for One Being More Accurate than the Other Introduction: The purpose of this experiment was to establish which method, a tin can with ice or a sling psychrometer, is more accurate at measuring dew point temperature. It was hypothesized that the sling psychrometer would be more accurate because it is a device solely designed to measure dew point temperature, whereas the tin can method uses a variety of materials assembled together. It was also predicted that the tin can and ice method would be less accurate because it dealt with more variables, which could contribute to potential sources of error. Humidity is a way of expressing the amount of water vapor present in the air. Two ways of articulating the water vapor content in the air are relative humidity and dew-point temperature. When the dew point and air temperature are equal, the air is saturated. An example of saturation is when water is trying to evaporate from a closed jar. Air pressure increases slowly as a result of the water vapor molecules being added to the air through evaporation. As the pressure in the air above the surface increases, some of the evaporating water is forced to return to the liquid. When the number of water vapor molecules returning to the liquid eventually balances out the number of water vapor molecules evaporating, it is said that the air is saturated. Saturation occurs when the air is holding the maximum amount of water vapor. Warm air provides a more conducive environment for water vapor because the water molecules have enough energy to remain in a gaseous form (2). Relative humidity, one of the ways of expressing humidity, measures a ratio of the amount of water vapor in the air compared to the total amount it can hold at that temperature. It is represented as a percent and always ranges from zero to one hundred. It cannot exceed one hundred percent because that is the point of saturation (3). Relative humidity does not tell how much water vapor is in the air, but rather how close the air is to saturation. It can be changed by adding or removing water vapor, or by changing temperature (2). A second way of representing humidity is with dew point temperature. Dew point temperature is the temperature at which something must be cooled in order to become saturated. This is because cold air holds less moisture than warm air. A higher dew point suggests a higher moisture content of air at a given temperature. Dew point temperature is never greater than the air temperature because, if it is equal to the air temperature, the air is saturated, but if the temperature dips below the dew point the water vapor will condense (1). Through the tin can, thermometer, and ice method of measuring dew point, condensation on the outside of the can was the indicator that the dew point had been reached. The tin can method works because, as ice is added to the water, the temperature of the water drops. As the temperature of the water drops, the temperature of the air above the surface drops as well. That being said, the air cannot hold as much water vapor because, with a decrease in temperature, there is a decrease in the amount of energy used to keep the water vapor in a gaseous state. As a result, the water vapor condenses and accumulates on the outside of the can. A sling psychrometer is another device used to measure the amount of water vapor in the air. It consists of two glass thermometers, one that measures the air temperature and another that measures the wet-bulb temperature. It works by having the wick dipped in water and it is then whirled around. As the device spins around, water evaporates from the wick on the wet bulb thermometer and cools the air temperature down. The cloth that surrounds the bulb on the sling psychrometer is already saturated with water. The process of evaporating air requires energy known as latent heat. Latent heat is the amount of heat released or absorbed by a substance when it changes phases. In this case, energy is absorbed during the vaporization of the water. This use of energy to vaporize the water causes the surrounding temperature to cool because it is losing energy in the form of heat. As the device is spun around, the water in the cloth around the bulb of the wet thermometer takes heat energy from the air to turn into a gas. This energy is necessary because more energy is needed for the water to transform into the gaseous state (4). The air eventually cools to a temperature low enough that it becomes saturated. On a sling psychrometer, the dew point is measured when the temperature stops decreasing. References: 1. "Observed Dew Point Temperature." WW2010. University of Illinois, 2010. Web. 21 Dec. 2011. <http://ww2010.atmos.uiuc.edu/(Gh)/guides/maps/sfcobs/ dwp.rxml>. 2. Tarbuck, Edward J, and Frederick K Lutgens. Earth Science. New Jersey: Prentice Hall, 2009. Print. 3. "Relative Humidity." WW2010. University of Illinois, 2010. Web. 21 Dec. 2011. <http://ww2010.atmos.uiuc.edu/(Gh)/guides/mtr/cld/dvlp/rh.rxml>. 4. Palmer, Chad. "How a Sling Psychrometer Works." USA Today: Weather. USA Today, 2008. Web. 21 Dec. 2011. <http://www.usatoday.com/weather/ wsling.htm>.