Lab #6
advertisement
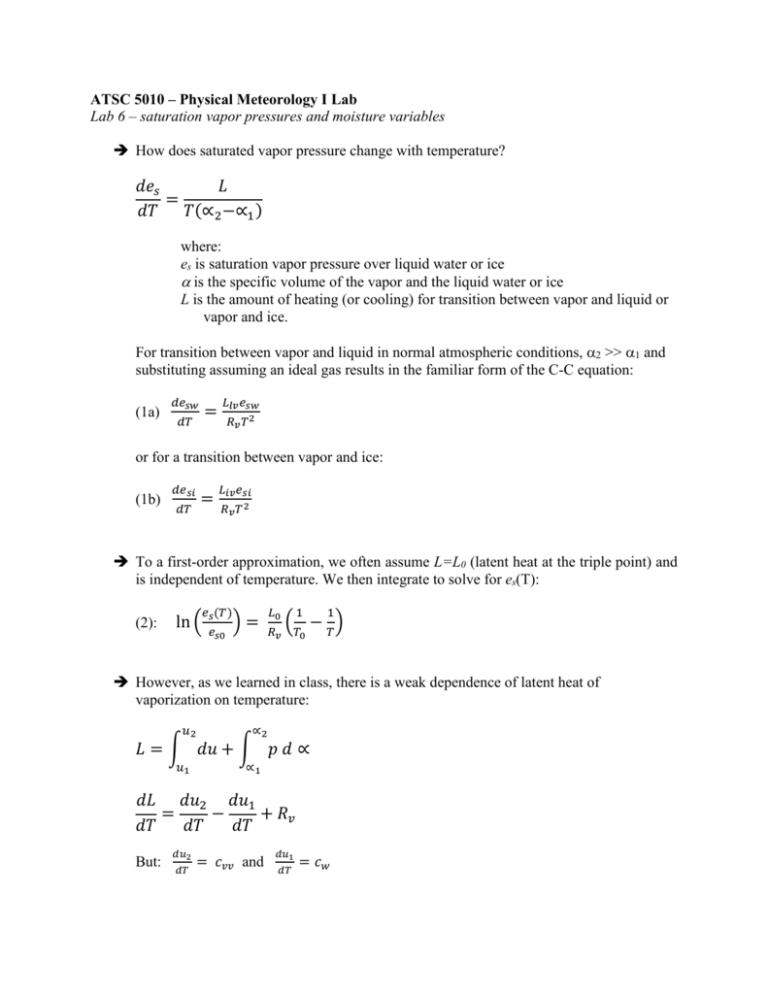
ATSC 5010 – Physical Meteorology I Lab Lab 6 – saturation vapor pressures and moisture variables How does saturated vapor pressure change with temperature? 𝑑𝑒𝑠 𝐿 = 𝑑𝑇 𝑇(∝2 −∝1 ) where: es is saturation vapor pressure over liquid water or ice is the specific volume of the vapor and the liquid water or ice L is the amount of heating (or cooling) for transition between vapor and liquid or vapor and ice. For transition between vapor and liquid in normal atmospheric conditions, 2 >> 1 and substituting assuming an ideal gas results in the familiar form of the C-C equation: (1a) 𝑑𝑒𝑠𝑤 = 𝑑𝑇 𝐿𝑙𝑣 𝑒𝑠𝑤 𝑅𝑣 𝑇 2 or for a transition between vapor and ice: (1b) 𝑑𝑒𝑠𝑖 = 𝑑𝑇 𝐿𝑖𝑣 𝑒𝑠𝑖 𝑅𝑣 𝑇 2 To a first-order approximation, we often assume L=L0 (latent heat at the triple point) and is independent of temperature. We then integrate to solve for es(T): (2): 𝑒𝑠 (𝑇) ln ( 𝑒𝑠0 )= 𝐿0 𝑅𝑣 1 1 (𝑇 − 𝑇) 0 However, as we learned in class, there is a weak dependence of latent heat of vaporization on temperature: 𝑢2 ∝2 𝐿 = ∫ 𝑑𝑢 + ∫ 𝑝 𝑑 ∝ 𝑢1 ∝1 𝑑𝐿 𝑑𝑢2 𝑑𝑢1 = − + 𝑅𝑣 𝑑𝑇 𝑑𝑇 𝑑𝑇 But: 𝑑𝑢2 𝑑𝑇 = 𝑐𝑣𝑣 and 𝑑𝑢1 𝑑𝑇 = 𝑐𝑤 where cw is specific heat capacity of liquid (for phase transition between vapor and ice we would consider ci). Thus: 𝑑𝐿 = 𝑐𝑣𝑣 − 𝑐𝑤 + 𝑅𝑣 = 𝑐𝑝𝑣 − 𝑐𝑤 𝑑𝑇 Integrating from some known state, L0 and T0: (3a): 𝐿𝑙𝑣 (𝑇) = 𝐿0 − (𝑐𝑤 − 𝑐𝑝𝑣 )(𝑇 − 𝑇0 ) (3b): 𝐿𝑖𝑣 (𝑇) = 𝐿0 − (𝑐𝑖 − 𝑐𝑝𝑣 )(𝑇 − 𝑇0 ) Equations 3 (a & b) work pretty well, but we are still ignoring the (slight) dependence of specific heat capacity on temperature. Ugh…… So, we are going to breakdown and consider an empirical function for es on temperature. Here we are ignoring the solution to C-C and computing directly esw (and esi) as a function of temperature. For saturation vapor pressure over liquid: 0.58002206 ∗ 104 𝐿𝑂𝐺(𝑒𝑠𝑤 ) = − + 0.13914993 ∗ 101 − (0.48640239 ∗ 10−1 )𝑇 𝑇 + (0.41764768 ∗ 10−4 )𝑇 2 − (0.14452093 ∗ 10−7 )𝑇 3 + (0.65459673 ∗ 101 ) ∗ 𝐿𝑂𝐺(𝑇) For saturation vapor pressure over ice: 0.56745359 ∗ 104 𝐿𝑂𝐺(𝑒𝑠𝑖 ) = − + 0.6392527 ∗ 101 − (0.96778430 ∗ 10−2 )𝑇 𝑇 + (0.62215701 ∗ 10−6 )𝑇 2 + (0.20747825 ∗ 10−8 )𝑇 3 − (0.94840240 ∗ 10−12 )𝑇 4 + (0.41635019 ∗ 101 ) ∗ 𝐿𝑂𝐺(𝑇) Exercise: Assume L0 = 2.500×106 J/kg at 0 C Assume es0 = 611.21 Pa at 0 C 1. Assuming no variation of L on temperature, solve (2) for esw(T) and for esi(T) 2. From the functional relationship in equation (3), substitute into the Clausius-Clapeyron equation and integrate. You will have a new, more complicated expression for es(T). 3. Build a function called sat_vapor that takes an input of temperature (vector) in Kelvin and returns a corresponding vector of saturation vapor pressures in Pascal. Allow your function to have keywords: ice, liquid, Lconst, Lfunction, polynomial. Upon calling sat_vapor, if keyword ‘ice’ is set, the saturation vapor pressure over ice is computed; likewise if keyword ‘liquid’ is set, the saturation vapor pressure over liquid is computed. If keyword ‘Lconst’ is set use the approximation that L is constant over the temperature range to compute saturation vapor pressure. If keyword ‘Lfunction’ is set use the functional relationship given in equations 3 (a & b) for latent heats to compute saturation vapor pressure. If keyword ‘polynomial’ is set, use the polynomial fit to compute directly the saturation vapor pressure. 4. Build a procedure (atsc5010_yourname_lab6) that produces a vector of temperatures from -40 to +100 C with 0.1 C resolution. In this procedure: a. Call sat_vapor several times, each time with the appropriate keyword(s) set to compute saturation vapor pressure over ice and/or over liquid for the temperature range from -40 to +100 C. b. Plot a graph (P-T diagram) showing the saturation vapor pressure over liquid and ice from -40 C to 100 C. Use red for ‘Lconstant’, blue for ‘Lfunction’, and black for ‘polynomial’. For saturation vapor pressure over liquid at temperatures below 0 C, use a dashed line. All other lines should be solid. c. Consider the comparison between ‘Lconstant’ and ‘polynomial’. For both saturation vapor pressure over liquid, plot the difference between the computed values using ‘Lconstant’ and ‘polynomial’ as a function of temperature. Do the same for saturation vapor pressure over ice. d. Similar to (c), Plot the percent difference between the two computations as a function of temperature (both for saturation vapor pressure over liquid and saturation vapor pressure over ice). e. Similar to (c) and (d), construct similar plots comparing the computations between ‘Lfunction’ and ‘polynomial_fit’. f. Now consider our most accurate model, ‘polynomial’, only. Noting that relative humidity wrt liquid, h, is defined as e/esw and relative humidity wrt ice, hi, is defined as e/esi. Consider a cloud that is composed of liquid water and ice crystals (mixed phase cloud). If the cloud is just saturated wrt to liquid, calculate (and plot) the relative humidity wrt ice as a function of temperature. Remember to use axis labels and titles on all your plots.