A. 1. Rock B. Out of roughly 30 mg... U, only one-third is still
advertisement
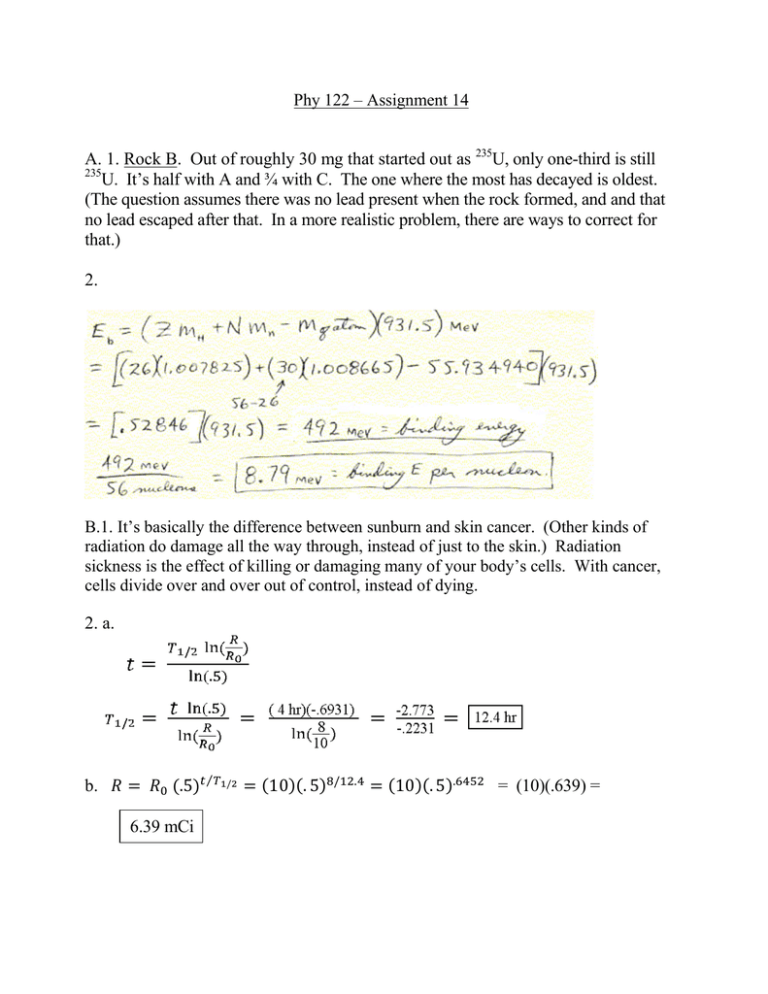
Phy 122 – Assignment 14 A. 1. Rock B. Out of roughly 30 mg that started out as 235U, only one-third is still 235 U. It’s half with A and ¾ with C. The one where the most has decayed is oldest. (The question assumes there was no lead present when the rock formed, and and that no lead escaped after that. In a more realistic problem, there are ways to correct for that.) 2. B.1. It’s basically the difference between sunburn and skin cancer. (Other kinds of radiation do damage all the way through, instead of just to the skin.) Radiation sickness is the effect of killing or damaging many of your body’s cells. With cancer, cells divide over and over out of control, instead of dying. 2. a. ⁄ b. 6.39 mCi = (10)(.639) = C.1. An alpha particle is a 4He nucleus. A beta particle is an electron. A gamma ray is a photon (electromagnetic radiation). 2. Fusion. (of hydrogen into helium) 3. For the decay to take place spontaneously, the disintegration energy, Q, must be positive. (Negative means energy must be put in for the process to happen.) Since Q = (total mass before – total mass after)c2 , just compare masses. D.1. Mass is equivalent to energy (E = mc2 ), and energy has to be supplied to pull a nucleus apart into individual paarticles. 2. There are several possible answers. The only one I mentioned in class is that neutrons feel the strong force, while neutrinos don’t. (And therefore, neutrinos interact very weakly with matter.) 3. The energy released is the difference in binding energy before vs. after. After: (100 nucleons)(about 8.7 MeV/nucleon) = 870 MeV So, the total for the two nuclei after is (2)(870) = 1740 MeV Before: (200 nucleons)(about 7.8 MeV/nucleon) = 1560 MeV About 1740 – about 1560 = about 180 MeV (If this was a quiz, I’d give full credit for 200 or 160, because anyone could be a little off reading the graph.) E. 1. Ten years is two half-lives. .80 2 = .40, .40 Twenty-five years is five half-lives. .80 .20 2 = .10, .10 2 = .05, .05 2 = .20 mCi 2 = .40, .40 2= 2 = .20, .025 mCi 2. Neutrons. 3. The mass numbers (on top) have to add up to the same thing on both sides of the arrow. The atomic numbers (bottom) have to add up to the same thing on both sides. The numbers shown below for the α, β, γ and antineutrino were not given in the question because you are supposed to know them. (a) : On top, 65 = 65 + 0. Bottom: 28 = 28 + 0. Is X also a Ni nucleus? Yes, because the atomic number didn’t change. (b) : Top: 215 = 211 + 4. Bottom: 84 = 82 + 2. Is X also a Po nucleus? No, because the atomic number changed. (c) ̅ : 208 = 208 + 0 + 0. 81 = 82 + (-1)+0. Is X also a Pb nucleus? No, because the atomic number changed. F.