Physics 43 HW 4
advertisement
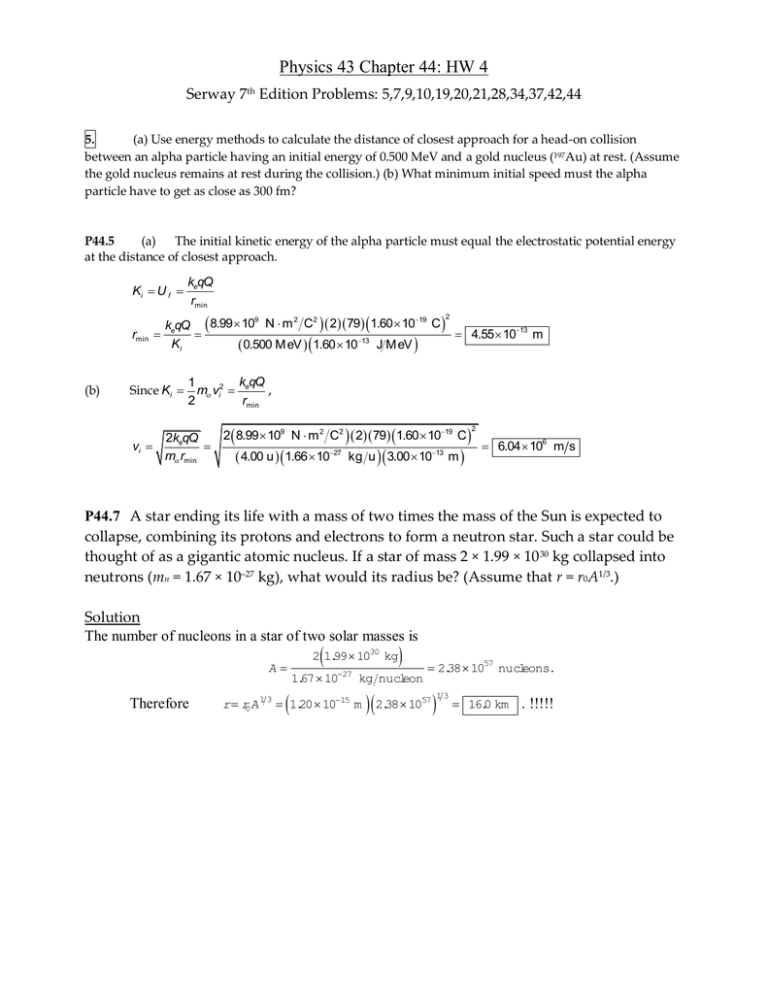
Physics 43 Chapter 44: HW 4 Serway 7th Edition Problems: 5,7,9,10,19,20,21,28,34,37,42,44 5. (a) Use energy methods to calculate the distance of closest approach for a head-on collision between an alpha particle having an initial energy of 0.500 MeV and a gold nucleus (197Au) at rest. (Assume the gold nucleus remains at rest during the collision.) (b) What minimum initial speed must the alpha particle have to get as close as 300 fm? P44.5 (a) The initial kinetic energy of the alpha particle must equal the electrostatic potential energy at the distance of closest approach. Ki U f keqQ rmin 9 2 2 19 k qQ 8.99 10 N m C 2 79 1.60 10 C e 4.55 1013 m Ki 0.500 M eV 1.60 1013 J M eV 2 rmin (b) Since Ki k qQ 1 m vi2 e , 2 rmin 2 8.99 109 N m 2 C2 2 79 1.60 1019 C 2keqQ vi 6.04 106 m s m rmin 4.00 u 1.66 1027 kg u 3.00 1013 m 2 P44.7 A star ending its life with a mass of two times the mass of the Sun is expected to collapse, combining its protons and electrons to form a neutron star. Such a star could be thought of as a gigantic atomic nucleus. If a star of mass 2 × 1.99 × 10 30 kg collapsed into neutrons (mn = 1.67 × 10–27 kg), what would its radius be? (Assume that r = r0A1/3.) Solution The number of nucleons in a star of two solar masses is A Therefore 2 1.99 1030 kg 27 1.67 10 kg nucleon r r0A 1 3 1.20 1015 m 2.38 1057 nucleons. 2.38 10 57 1 3 16.0 km . !!!!! 9. Calculate the binding energy per nucleon for (a) 2H, (b) 4He, (c) 56Fe, and (d) 238U. Eb = (Zmp + Nmn – MA) x 931.494 MeV/u Using atomic masses as given in the table in the text, 2.014 102 11.008 665 11.007 825 (a) For 21 H : (b) For 42 He : (c) For 56 26 Fe : 2 Eb 931.5 MeV 0.001194 u 1.11 MeV nucleon A u 2 1.008 665 2 1.007 825 4.002 603 4 Eb 2 0.007 59 u c 7.07 MeV nucleon A 301.008 665 261.007 825 55.934 942 0.528 u Eb 0.528 0.009 44 u c2 8.79 MeV nucleon A 56 (d) 1461.008 665 921.007 825 238.050 783 1.934 2 u For 238 92 U : Eb 1.934 2 0.008 13 u c2 7.57 MeV nucleon A 238 10. The iron isotope 56Fe is near the peak of the stability curve. This is why iron is generally prominent in the spectrum of the Sun and stars. Show that 56Fe has a higher binding energy per nucleon than its neighbors 55Mn and 59Co. Compare your results with Figure 44.5. M ZmH Nmn M *P44.10 Nuclei Z N M in u M in u Eb in MeV A 8.765 8.790 8.768 Eb M 931.5 A A 25 30 54.938 050 0.517 5 26 30 55.934 942 0.528 46 27 32 58.933 200 0.555 35 E 56 Fe has a greater b than its neighbors. This tells us finer detail than is shown in Figure 44.5. A 55 Mn Fe 59 Co 56 A sample of radioactive material contains 1.00 × 1015 atoms and has an activity of 6.00 × 1011 Bq. What is its half-life? 19 dN N dt T1 2 ln 2 1 dN 15 6.00 1011 6.00 104 s1 1.00 10 N dt so 1.16 103 s 19.3 m in 20. The half-life of 131I is 8.04 days. On a certain day, the activity of an iodine-131 sample is 6.40 mCi. What is its activity 40.2 days later? 5 1 6.40 mCi e ln 2 6.40 mCi 5 0.200 mCi 2 P44.20 R R0 e t 6.40 mCi e ln 2 8.04 d 40.2 d A freshly prepared sample of a certain radioactive isotope has an activity of 10.0 mCi. After 4.00 h, its activity is 8.00 mCi. (a) Find the decay constant and half-life. (b) How many atoms of the isotope were contained in the freshly prepared sample? (c) What is the sample’s activity 30.0 h after it is prepared? 21 Solution (a) R R0e t , From 1 R 1 10.0 2 1 5 1 ln 0 ln 5.58 10 h 1.55 10 s t R 4.00 h 8.00 T1 2 R0 12.4 h 10.0 103 Ci 3.70 1010 1 Ci 1.55 105 s s 13 2.39 10 atom s (b) N0 (c) R R0e t 10.0 m Ci exp 5.58 102 30.0 1.88 m Ci ln 2 28. Identify the missing nuclide or particle (X): (a) X (b) 215 84 109 48 (e) 14 7 Ni X= Po X (c) X (d) 65 28 55 26 65 28 X Ni 211 82 Pb Fe e v X= Cd X 109 47 Ag v N 42 He X 17 8 O 55 27 Co X= X= 0 1 1 1 e H 28 solution: (a) A gamma ray has zero charge and it contains no protons or neutrons. So for a gamma ray Z 0 and A 0 . Keeping the total values of Z and A for the system conserved then requires Z 28 and A 65 for X. With this atomic number it must be nickel, and the nucleus must be in an exited state, so it is 65 28 Ni . 42 He has (b) so for X for Pb Z 2 and A4 we require Z 84 2 82 and A 215 4 211 , X 211 82 Pb (c) A positron e 01 e has charge the same as a nucleus with Z 1 . A neutrino 00 has no charge. Neither contains any protons or neutrons. So X must have by conservation Z 26 1 27 . It is Co. 55 And A 55 0 55 . It is 27 Co . Similar reasoning about balancing the sums of Z and A across the reaction reveals: (d) 0 1 e 1 (e) 1 H (or p). Note that this process is a nuclear reaction, rather than radioactive decay. We can solve it from the same principles, which are fundamentally conservation of charge and conservation of baryon number. 34. Determine which decays can occur spontaneously: Ca e (a) 40 20 (b) 98 44 144 60 (c) 40 19 K Ru 42 He 94 42 Mo 4 Nd 2 He 140 58 Ce P44.34 (a) For e decay, Q M X M Y 2me c2 39.962 591 u 39.963 999 u 2 0.000 549 u 931.5 MeV u Q 2.33 MeV Since Q 0 , the decay cannot occur (b) spontaneously. For alpha decay, Q M X M M Y c2 97.905 287 u 4.002 603 u 93.905 088 u 931.5 MeV u Q 2.24 MeV Since Q 0 , the decay cannot occur (c) spontaneously. For alpha decay, Q M X M M Y c2 143.910 083 u 4.002 603 u 139.905 434 u 931.5 MeV u Q 1.91 MeV Since Q 0 , the decay can occur spontaneously. 37. Indoor air pollution. Uranium is naturally present in rock and soil. At one step in its series of radioactive decays, 238U produces the chemically inert gas radon-222, with a half-life of 3.82 days. The radon seeps out of the ground to mix into the atmosphere, typically making open air radioactive with activity 0.3 pCi/L. In homes 222Rn can be a serious pollutant, accumulating to reach much higher activities in enclosed spaces. If the radon radioactivity exceeds 4 pCi/L, the Environmental Protection Agency suggests taking action to reduce it, such as by reducing infiltration of air from the ground. (a) Convert the activity 4 pCi/L to units of becquerel per cubic meter. (b) How many 222Rn atoms are in one cubic meter of air displaying this activity? (c) What fraction of the mass of the air does the radon constitute? P44.37 N (b) 4.00 1012 Ci 3.70 1010 Bq 1.00 103 L 3 4.00 pCi L 148 Bq m 1L 1 Ci 1 m3 (a) T1 2 3 3.82 d 86 400 s 7 3 R 148 Bq m 7.05 10 atoms m ln 2 ln2 1 d R 1 mol 222 g 14 3 mass 7.05 107 atoms m 3 2.60 10 g m 23 6.02 10 atoms 1 mol Since air has a density of 1.20 kg m 3 , the fraction consisting of radon is (c) fraction 42. 2.60 1014 g m 3 2.17 1017 1 200 g m 3 A beam of 6.61-MeV protons is incident on a target of p 27 ( 14 27 13 Al 27 14 27 13 Al . Those that collide produce the reaction Si n Si has mass 26.986 705 u.) Ignoring any recoil of the product nucleus, determine the kinetic energy of the emerging neutrons. P44.42 Neglect recoil of product nucleus (i.e., do not require momentum conservation for the system of colliding particles). The energy balance gives Kemerging Kincident Q . To find Q: Q M H M Al M Si mn c2 Q 1.007 825 26.981 539 26.986 705 1.008 665 u 931.5 MeV u 5.59 MeV Thus, 44. (a) Suppose 10 5 Kemerging 6.61 MeV 5.59 MeV 1.02 MeV . B is struck by an alpha particle, releasing a proton and a product nucleus in the reaction. What is the product nucleus? (b) An alpha particle and a product nucleus are produced when is struck by a proton. What is the product nucleus? P44.44 (a) 10 5 B 42 He 136 C 11 H The product nucleus is (b) 13 6 13 6 C . 10 5 B . C 11 H 105 B 42 He The product nucleus is 13 6 C








