doc
advertisement

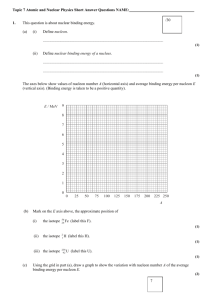
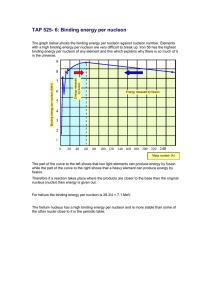
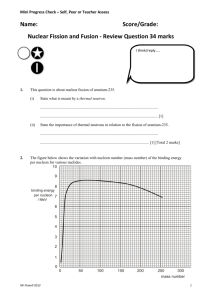
PHYS212-071 HW # 11 (Chapter 13) (Numbers refer to 2nd Edition of Textbook) 15. Calculate the binding energy per nucleon for the nuclei 20 40 93 (a)10 Ne, (b) 20 Ca, (c) 41 Nb, (d )197 79 Au Answer: BE / A Zm p ( A Z )mn M ( ZAX ) 931.4943 / A Ne-20: 8.03 MeV/nucleon Ca-40: 8.55 MeV/nucleon Nb-93: 8.66 MeV/nucleon Au-197: 7.92 MeV/nucleon 22. The half-life of 131 I is 8.04 days. (a) Calculate the decay constant for this isotope. (b) Find the number of I nuclei necessary to produce a sample with an activity of 0.5 Ci. 131 Answer: (a) = 0.693/T1/2 = 0.693/ (8.04 x 24 x 3600) s-1 = 9.97 x 10-7 s-1 (b) A = N N = A/ = (0.5 x 10-6 x 3.7 x 1010)/ (9.97 x10-7) 2 x 1010 nuclei 26. How many radioactive atoms are present in a sample that has an activity of 0.2 Ci and a half-life of 8.1 days? Answer: N = A/ = A x T1/2/0.693 = (0.2 x 10-6 x 3.7 x 1010) x (8.1 x 24 x 3600)/0.693 8 x109 41. Find the energy released in the alpha decay of 238 92 U : 4 U 234 90Th 2 He 238 92 Answer: If we neglect the recoil energy of the heavy Thorium nucleus, then the energy of the alpha particle is just the Q of the reaction; namely Q = (M (U-238) – M (Th-234) – M (He-4)) x 931.4943 MeV = 4.3 MeV 1 50. Starting with 235 92 U , the sequence of decays shown in Figure P13.50 is observed, ending with the stable isotope 207 82 Pb . Enter the correct isotope symbol in each open square. 2