Chapter 22 QQ

QUICK QUIZ 22.1
(end of section 22.1)
An engine cycle is shown on the PV diagram below, with some of the energy transfers indicated on the diagram. The engine absorbs heat energy, | Q h
|, along the path from A to B, and then outputs an amount of work along the path from B to
C that is a) less than | Q h d) a or b, or e) a, b or c.
|, b) equal to | Q h
|, c) greater than | Q h
|,
QUICK QUIZ 22.1 ANSWER
(e) The second law refers to the energy transfers that occur during an entire cycle. For the entire cycle, it is impossible to only absorb heat | Q h
| at the hot reservoir and convert that heat entirely to work. In addition, from the first law, the net heat absorbed must be equal to the net work output for the entire cycle. However, it is possible to absorb a certain quantity of heat along one branch of the cycle and output a quantity of work that is less than, greater than, or equal to this heat energy along some other branch of the cycle. For example, the cycle shown approximates two isotherms (A to B and C to D) and two adiabats (D to A and B to C). Recall from Equation 20.13 that the heat absorbed along the isotherm from A to B will be | Q h
| = nRT h ln( V
B
/ V
A
).
In addition, the work output along the reversible adiabat from B to C will be equal to the change in internal energy of the gas. From Chapter 21 for a monatomic ideal gas, this quantity will be W =
D
E int
= (3/2) nR ( T h
– T c
).
Depending on the relative values, V
A
, V
B
, T h and T c
, the work may be greater than, less than or equal to the heat absorbed.
QUICK QUIZ 22.2
(end of section 22.2)
The second law implies that the coefficient of performance of a heat pump a) must be less than one, b) must be less than or equal to one, c) can not be less than one, d) can not be infinite, or e) can not be zero.
QUICK QUIZ 22.2 ANSWER
(d).
According to Equation 22.3, the coefficient of performance for a heat pump is equal to the energy transferred at high temperature divided by the work done by the pump. Violating the second law would imply that heat is transferred at high temperature with no input of work. Therefore, the denominator would be zero and the coefficient of performance would be infinite. It is possible to have a heat pump with a coefficient of performance less than one. This implies that the input work is transferred into heat that goes both in the hot and the cold reservoir. As discussed in the text, such a situation can occur if the outside temperature is very low.
QUICK QUIZ 22.3
(end of section 22.4)
In the argument for the validity of Carnot’s theorem, t he primary quality used to distinguish a Carnot engine from a real engine is that a) a Carnot engine operates in a cycle of two adiabats and two isotherms, b) a Carnot engine is
100% efficient, c) a Carnot engine is reversible, or d) a
Carnot engine does not deliver heat to a cold reservoir.
QUICK QUIZ 22.3 ANSWER
(c).
In the argument, a real engine, with assumed efficiency that is greater than a
Carnot engine ’ s efficiency, is used to drive a Carnot engine in reverse. Because a
Carnot engine is reversible, the heat taken from the hot reservoir for the Carnot engine will be precisely the same as the heat delivered to the hot reservoir for the
Carnot refrigerator. This reversible quality of the Carnot engine is the main feature of the argument. From the inequality e > e
C
, or | W |/| Q
’ h
| > | W |/| Q h
|, one can then establish that | Q h
| > | Q ’ h
| and that there will be a net heat transfer to the hot reservoir with no net input of work, thus defying the second law.
QUICK QUIZ 22.4
(end of section 22.5)
As an automotive engineer, you are given the choice of increasing the efficiency of a gasoline engine by increasing the compression ratio by a factor of two or by increasing the absolute highest temperature ( T
C
) by a factor of two. To get the highest efficiency, you should a) increase the compression ratio, b) increase T
C
, c) either method will work equally well, or d) more information is needed.
QUICK QUIZ 22.4 ANSWER
(b).
Example 22.6 gives the efficiency in terms of the compression ratio, r =
V
1
/ V
2
, and the high temperature, T
C values, r o and T o
. Replacing these variables with their initial
, and assuming g = 1.4, we obtain for the initial efficiency, e o
1
( V V
1 2
) g
1
1
( ) o
0.4
1
T
D 1
T
C
T
T
D o
If we double the compression ratio, we obtain e
1 r o
1
0.4
1
0.4
2 ( ) o
0.4
T
D
2
0.4
T o
On the other hand, if we double the high temperature, we obtain e
2
T
D
2 T o
The efficiency will be greater in the second case since the denominator of the fraction will be larger.
QUICK QUIZ 22.5
(end of section 22.7)
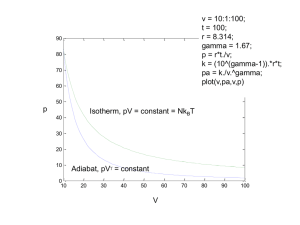
The green curve in the PV diagram below represents a reversible adiabatic expansion of an ideal gas from some initial volume, V i volume, V f and pressure, P f
, and Pressure, P i
, to some final
. If the same gas starting from the same initial volume and pressure were to undergo an adiabatic free expansion to the same final volume, V f
, the point on the diagram that could represent the final state would be a) point A, b) point B (the same as the final state for the reversible adiabatic expansion), c) point C, or d) points A, B, or C, depending on the situation.
QUICK QUIZ 22.5 ANSWER
(a). Recall from the discussion in Section 22.7 that an adiabatic free expansion of an ideal gas is an isothermal process. In fact, to determine the change in entropy for this process, a reversible isotherm was used. For an ideal gas, reversible isothermal processes are represented by curves on a PV diagram that have the form, PV = constant. In addition, reversible adiabatic processes have the form, PV g = constant, and are therefore always steeper curves than the isotherms, as examination of Figure 22.11 reveals. The less steep isotherm (shown in red) starting from the same initial pressure and volume, must end up at a pressure that is higher than the final pressure of the reversible adiabat. Also, a reversible adiabat for a higher constant entropy is shown in purple. The final state, A, sits on this curve of higher entropy, consistent with the fact that the entropy increases in an adiabatic free expansion.