Reversibility
advertisement
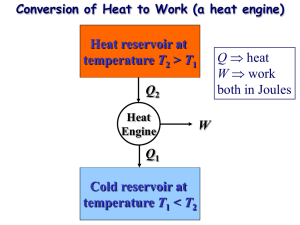
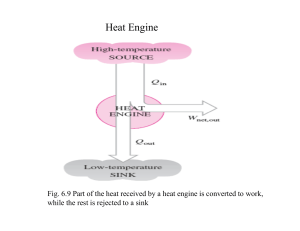
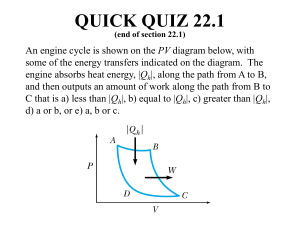
Reversibility Reversible Process Quasi-static processes meant that each step was slo enough to maintain equilibrium. If the process is reversed the work and heat also just reverse sign. Heat lost to friction is not reversible. Reversible process is infinitely slow; system stays at equilibrium. Irreversible process has heat loss to friction; system has net loss of useful energy. Plotting Reversibility The PV diagram can plot a quasi-static, reversible process. Irreversible processes cannot be plotted. P P P2 ,V2 , T2 P2 ,V2 , T2 P1 ,V1 , T1 P1 ,V1 , T1 V V Reversible Cycle A series of reversible processes can be plotted on a PV diagram. A reversible cycle is a set of reversible processes that return to the initial state. P P1 ,V1 , T1 P3 ,V3 , T3 P2 ,V2 , T2 V Carnot Engine An ideal Carnot engine consists of four processes. 1) expand gas isothermally. 2) expand gas adiabatically. 3) compress gas isothermally. 4) compress gas adiabatically. Expansion During the isothermal expansion there is work done with heat in. W12 QH nRTH ln V2 V1 There is no heat flow during the adiabatic expansion, but work is done. P P1 ,V1 , T1 P2 ,V2 , T2 P3 ,V3 , T3 P2V2 CP / CV P3V3 CP / CV V Compression During the isothermal compression work is returned with heat out. W34 QL nRTL ln V3 V4 P P1 ,V1 , T1 There is no heat flow during the adiabatic compression, but work is returned. P4V4 C P / CV P1V1 C P / CV P4 ,V4 , T4 P3 ,V3 , T3 V Carnot Efficiency The heat and temperatures are related in a Carnot engine. • |QL| / |QH| = TL / TH This is the ideal engine efficiency. eideal 1 TL TH Underwater A nuclear plant produces 540 MW of power while the fuel releases 1590 MW. Steam enters the turbine at 556 K and is discharged at 313 K. What is the ideal and actual efficiency? The ideal efficiency assumes a Carnot engine. T eideal 1 L 43.7% TH The actual efficiency is found from the power usage. e W QH 540 MW - s 34% 1590 MW - s next