η 6-7 THE CARNOT CYCLE 6-8 CARNOT PRINCIPLES
advertisement

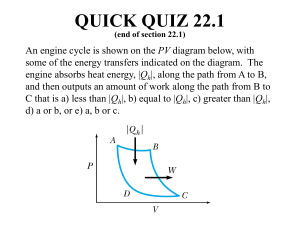
The Carnot Cycle (Heat Engine) 6-7 THE CARNOT CYCLE The Reversed Carnot Cycle (Refrigerator, Heat Pump) P P TH 1 TH 1 QH QH 2 4 TH Q=0 Wnet ,out Wnet ,in Q=0 Q=0 4 2 3 QL Sadi Carnot (1822-1888) V1 The Carnot Cycle consists V4 V2 Reversible Processes V3 V1 V2 W 2 V4 TL V V3 TL 3 V3 2 V2 3 V3 4 V4 continues to expand -Q -W 3 QL V gas 1 Q TL TL of 4 reversible processes 1 TH Q=0 2 V2 2 • the paths coinside 4 V4 1 V1 • heat transfer is due to infinitely small difference in temperature • at thermal equilibrium V1 1 gas Irreversible Processes ∆T > 0 insulated insulated QH • heat transfer is due to finite difference in temperature • heat generation by friction 6-8 CARNOT PRINCIPLES QL Isothermal expansion at TH heat QH is added Adiabatic expansion Q=0 Temperature dropped to TL Isothermal compression Adiabatic compression at TL Q=0 Temperature raised to TH heat QL is rejected Efficiency of two Heat Engines operating between the same two reservoirs at TL and TH TH C1 η irreversible < η reversible η IR < η R Violation of Carnot Principles yields violation of the IR 2nd Law of Thermodynamics C2 η R1 = η R2 R1 R2 η reversible 1 = η reversible 2 TL 6-9 THE THERMODYNAMIC TEMPERATURE SCALE For reversible heat engine operating between TL and TH : QL T = L QH TH Kelvin Scale of Absolute Temperature 6-10 THE CARNOT HEAT ENGINE The efficiency of heat engine operating on a reversible Carnot cycle between TL and TH : ηC = 1 − 6-11 THE CARNOT HEAT PUMP QL T = 1 − L QH TH Carnot Heat Pump: COPCHP = 1 Q 1− L QH Carnot Efficiency ηC is the highest possible efficiency of the heat engine operating between two reservoirs at TL and TH Carnot Refrigerator: = 1 T 1− L TH COPCR = 1 1 = QH TH −1 −1 QL TL INSTITUT NATIONAL DES SCIENCES APPLIQUEES, LYON