Problem Set 3 2004
advertisement

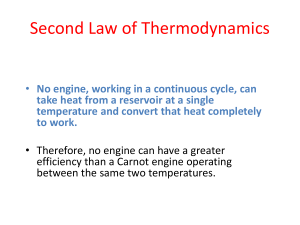
2TP - Thermal Physics Department of Physics, University of Surrey Problem Set 3: Internal Energy and Carnot Engines You should read Chapters 3 and 4 of Thermal Physics by C.B.P. Finn. (1) Use the first law of thermodynamics to show that in an isothermal expansion of an ideal gas the work done by the gas equals the heat absorbed. (2) Explain why in a free adiabatic expansion of an ideal gas from a volume of V1 to a volume of V2 the temperature of the gas is unchanged. (3) Two moles of a monoatomic ideal gas are at a temperature of 300 K. The gas expands reversibly and isothermally to twice its original volume. Calculate the work done by the gas, the heat supplied, and the change in internal energy. (4) Using the first law of thermodynamics, find the change in the internal energy (U) of one mole of a monoatomic ideal gas in an isobaric expansion at 1 atm from a volume of 5 m3 to a volume of 10 m3. Then find U using the definition of Cv. Does your answer depend on the number of moles of gas? (5) A system is taken from a to b via c, as is shown on the diagram below. On going from a to b, 80 J of heat flows into the system, and it does 30 J of work on the surroundings. (a) When the system goes from a to b via d, calculate the amount of heat flowing into the system if the work done on the surroundings is 10 J. (b) If the system goes from b to a via the irregular path shown on the diagram when 20 J of work is done on it, calculate the amount of heat absorbed. (c) If the internal energy of the system at point a is 0 J and the internal energy at d is 40 J, find the heat absorbed along the paths ad and db. For discussion during Tutorial 3 in Week 6 on 9 November, 2011 2TP - Thermal Physics Department of Physics, University of Surrey (6) One mole of a monoatomic ideal gas is at a temperature of 400 K. The gas expands reversibly and isothermally to three times its original volume. What is the work done by the gas? How much heat is absorbed by the gas? (7) Derive an expression for the work done by an ideal gas during a reversible adiabatic expansion from an initial pressure of P1 and volume V1 to final values of P2 and V2. (8) Heat is supplied to an engine at the rate of 106 Jmin-1, and the engine has an output of 10 horsepower. What is the efficiency of the engine? What is its heat output per minute? (Note that 1 Hp = 746 W.) (9) To increase the efficiency of a Carnot engine, is it better to increase the temperature of the hot reservoir by 10 K (keeping the temperature of the cold reservoir constant) or to decrease the cold reservoir by 10 K (keeping the temperature of the hot reservoir constant)? Support your answer with calculations. (10) A steam engine takes in superheated steam at 270 ºC and discharges water from its cylinder at 50 ºC. What is the efficiency of a Carnot engine operating between these temperatures? Assume that the efficiency of the steam engine is 30% and that its useful power output is 200 kW. In one day how much heat does the engine absorb from the hot reservoir and how much heat does it discharge to the cold reservoir? Adiabatic Expansion and Compression of an Ideal Gas (11) A spherical interstellar cloud, consisting of an ideal gas with = 5/2, collapses adiabatically from a radius of 1013 m to a radius of 1012 m. If the initial temperature of the cloud is 10 K, what is its temperature after its collapse? (12) Given that PV is a constant for a reversible, adiabatic expansion of an ideal gas, show that TV-1 is also a constant. (13) The fireball of a uranium fission bomb consists of a sphere of gas of radius 15 m and a temperature of 300,000 K shortly after detonation. Assuming that the expansion is adiabatic and that the fireball remains spherical, estimate the radius of the fireball when its temperature is 3000 K. Assume that = 1.4 (14) A spherical interstellar cloud, consisting mainly of helium gas (which is monoatomic), collapses adiabatically from a radius of 1013 m to a radius of 1012 m. By what factor does the pressure change in the cloud during the process? You can assume that Cp/Cv = 5/3. For discussion during Tutorial 3 in Week 6 on 9 November, 2011