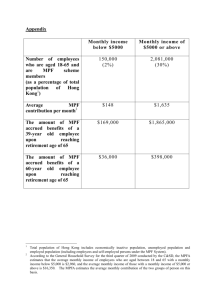
Biochemical Control of the Cell Cycle

Biochemical Control of the
Cell Cycle
BNS230
Lecture programme
• Three lectures
• Aims
– Describe the cell cycle
– Discuss the importance of the cell cycle
– Discuss how the cycle is regulated
12 hours
Cell division
15 hours
M phase
G
2 phase
G
1 phase
S-phase
(DNA synthesis
G
0 state
16 hour cell cycle
5 hours
Cell cycle definition
• A series of distinct biochemical and physiological events occurring during replication of a cell
• Occurs in eukaryotes
• Does not occur in prokaryotes
• Time of cell cycle is variable
Cell cycle timing
• Yeast 120 minutes (rich medium)
• Insect embryos 15-30 minutes
• Plant and mammals 15-20 hours
• Some adults don’t divide
– Terminally differentiated
– e.g. Nerve cells, eye lens
• Some quiescent unless activated
– Fibroblasts in wound healing
Components of the cell cycle
• M phase
– Cell division
– Divided into six phases
• Prophase
• Prometaphase
• Metaphase
• Anaphase
• Telophase
• Cytokinesis
Components of the cell cycle
• G1 phase
– Cell checks everything OK for DNA replication
– Accumulates signals that activate replication
– Chloroplast and mitochondria division not linked to cell cycle
Components of the cell cycle
• S-phase
– The chromosomes replicate
– Two daughter chromosomes are called chromatids
– Joined at centromere
– Number of chromosomes in diploid is four
Components of the cell cycle
• G2-phase
– Cell checks everything is OK for cell division
– Accumulates proteins that activate cell division
Why have a cell cycle?
• Comprises gaps and distinct phases of
DNA replication and cell division
• If replicating DNA is forced to condense
(as in mitosis) they fragment
• Similarly if replication before mitosis
– Unequal genetic seperation
• I.e. Important to keep DNA replication and mitosis separate
Why have a cell cycle?
• Important to have divisions in mitosis
• e.g. Important metaphase complete before anaphase. Why?
• If not segregation of chromosomes before attachment of chromatids to microtubles in opposite poles is possible
• Down syndrome due to extra chromosome 21
Why have a cell cycle?
• Gaps provide cell with chance to assess its status prior to DNA replication or cell division
• During the cell cycle there are several checks to monitor status
• These are called checkpoints
Checkpoints
• Checkpoint if G1 monitors size of cell in budding yeast ( Saccharomyces cerevisae )
• At certain size cell becomes committed to DNA replication
• Called start or replication site
Evidence of size checkpoint
• Yeast cells (budding yeast) grown in rich medium
• Switch to minimal medium
• Cells recently entering G1 (buds) delayed in G1 (longer to enter S-phase)
• Large cells above threshold size still go to S-phase at same time as in rich medium
Evidence of size checkpoint
• Yeast in rich medium
– 120 minute cell cycle
• Short G1 phase
• Yeast in minimal medium
– Eight hour cell cycle primarily because of long G1 phase
Checkpoints
• Checkpoint 2 in G1 monitors DNA damage
• Evidence?
– Expose cells to mutagen or irradiation
– Cell cycle arrest in either G1 phase or G2 phase
• The protein p53 involved in cell cycle arrest
– Tumour suppresser
Checkpoints
• Checkpoint in S-phase monitors completion of DNA replication
– Cell does not enter M-phase until DNA synthesis is complete
• Checkpoint in G2
– DNA breaks cause arrest
– Otherwise when chromosomes segregate in mitosis DNA distal to breaak won’t segregate
Checkpoints
• Checkpoint in mitosis
– Senses when mitotic spindles have not formed
– Arrests in M-phase
– Otherwise unequal segregation of chromosomes into daughter cells
• Described cell cycle, now I will talk about genes and proteins that control this process
Molecular control of cell cycle
• Two experimental approaches
– Biochemical
• Sea urchin fertilised eggs
• Rapid
• Synchronous division
• Analyse proteins at various stages of cycle
– Genetic analysis using
• Budding yeast Saccharomyces cerevisae
• Fission yeast Schizosaccharomyces pombe
Using genetics to study the cell cycle
• To study the genetic basis of a biological event
– Make mutants defective in that event
– Determine which genes have been mutated
– Understand role of gene (and encoded protein) in the event
– Problem: How do you make mutants that disrupt the cell cycle
– Cells will not replicate
Using genetics to study the cell cycle
• Isolate temperature sensitive mutants that have defect in cell cycle
• At low temperature these mutants progress through cell cycle
• Arrest in cell cycle at elevated temperature
• Mutation causes gene product (protein) to be highly sensitive to temperature
Using genetics to study the cell cycle
• Isolation of genes that regulate the cell cycle
• Step 1: Create strains with mutations in cell cycle genes
Isolating cell cycle mutants
Yeast culture
(S. pombe)
Mutagenise and plate out at high and low temperature
37 °C
30 °C
Colonies 4 and 10 are possible cell cycle mutants. Called cell division cycle (cdc) mutants >70 cdc mutants isolated
Are the temperature sensitive mutants cdc mutants?
Grow colonies at 30 °C
Shift temperature to 37 °C
Look under a microscope
Colony 4: Too small; enters mitosis too early (Wee 1 mutant)
Colony 10: very long stuck in G2
(cdc25 mutant)
Wild type cells
Using genetics to study the cell cycle
• Step 2: Insert plasmids containing fragments of wild type DNA
• Step 3: Look for plasmid that corrects genetic defects
• Step 4: Plasmid contains a cell cycle control gene
What do we do with the mutants?
Wild type
S. pombe
Use mutants to isolate cdc genes and then study what the proteins do
Extract DNA
Yeast vector
Wee1 cdc25
Cut with restriction enzyme and ligate into vector
Take recombinant vectors and transform into cdc mutants
• Wee mutant with normal gene wee1 gene in plasmid will grow at 37
• cdc25 mutant with normal cdc25 gene in plasmid will grow at 37
• I.e gene in recombinant plasmid is complementing the mutation
Biochemical studies
• 1st evidence proteins regulate cell cycle
– Fuse interphase cells (G1, S or G2) withMphase cells
– Cell membranes breakdown and chromosomes condense
– I.e Mitotic cells produce proteins that cause mitotic changes in other cells
Microinjection with frog oocyte
• Oocyte stays in G2-phase
• Male gets busy and female produces progesterone
• Oocyte enters mitosis
• Purify proteins from oocyte cells treated with progesterone
• Inject into G2 arrested cells and see which protein causes mitosis (1971)
MPF
• Protein identified that causes mitosis
• Called maturation promoting factor
• MPF in all mitotic cells from yeast to humans
• Renamed mitosis-promoting factor
Properties of MPF
• MPF activity changes through the cell cycle
• MPF activity appears at the G2/M interphase
• and then rapidly decrease
How does MPF cause mitosis?
• It’s a protein kinase
– Phosphorylates proteins
• Phosphorylates proteins involved in mitosis
• Phosphorylates histones causing chromatin condensation
• Phosphorylates nuclear membrane proteins (lamins) causing membrane disruption
Characterisation of MPF
• Consists of two subunits; A and B
• Subunit A: Protein kinase
• Subunit B: Regulatory polypeptide called cyclin B
• Protein kinase present throughout cell cycle
• Cyclin B gradually increases during interphase (G1, S, G2)
• Cyclin B falls abruptly in anaphase
(mid-mitosis)
What does this profile tell you?
MPF not just due to association of subunits A and B other factors involved
Protein kinase (subunit A)
Cyclin B levels (subunit B)
MPF activity
G1 S G2 M
Prophase
MPF}
Cyclin B (subunit B)
Protein kinase (subunit A)
Metaphase
Ubiquitin
Anaphase
Interphase
(G1-S-G2)
Telephase
Proteosome
Cyclin B
• How do Cyclin B levels decrease abruptly
• Proteolytic degradation
• Degraded in a protease complex present in eukaryotic cells called “The
Proteosome”
• Specific proteins degraded by complex when tagged by a small peptide called ubiquitin
Cyclin B
• Cyclin B is tagged for Proteosome degradation at anaphase
– Tagged at N-terminus at sequence called
– Destruction box
– DBRP binds to Destruction box
• Guides Ubiquitin ligase to add ubiquitin molecules to Cyclin B
• Why is Cyclin B only degraded in anaphase
Destruction box
P
P
Protein de-phosphorylase
DBRP
(active)
MPF?
DBRP
(inactive)
Ubiquitin ligase adds ubiquitin when DBRP binds to the destruction box
DBRP = Destruction box recognition protein
Cyclin B
• DBRP is normally inactive and is only activated in anaphase via phosphorylation
• Possible MPF phosphorylates DBRP causing
Cyclin B destruction
– Binds to the destruction box
– Activates ubiquitin ligase to add ubiquitin to
Cyclin B
– Cyclin B then targeted to the Proteosome for degradation
Cyclin B
• When this causes MPF inactivation
– DBRP dephosphorylated by constitutive phosphorylase
• Other proteins also control MPF
– Activity doesn’t increase as Cyclin B increases
• Proteins discovered in yeast by cdc mutant complementation
inactive
Protein kinase (subunit A) cdc2
MPF}
Y15 T161
Wee1 cdc13
Cyclin B (subunit B)
Inactive MPF
P
CAK
Y15 T161
Inactive MPF
P Y15 T161 cdc25
P
Active MPF
Y15 T161 P
MPF activity
• Wee mutant small: Enters mitosis prematurely
• cdc 25 mutant long: Stays in G2 for longer
• Wee phosphorylates Y15 and inactivates MPF
• CAK (cdc2 [MPF]-activating kinase) phosphorylates T161
• cdc25 dephosphorylates Y15 and activates MPF
Cell cycle
• How is entry into S-phase controlled?
• Throughout cell cycle the protein kinase
(cdc28 in sc and cdc2 in sp) binds to specific cyclins
• This changes the specificity of the protein kinase
Activity of Protein Kinase
• Cdc28-cyclins B1-4: Protein kinase activates proteins involved in early mitosis by phorphorylating them
• Cdc28-cyclins 1-3: Protein kinase activates proteins involved in initiation of
DNA replication by phosphorylating them
• cdc28-cyclin 5: Phorphorylates and thus activates proteins that maintain DNA replication
How many protein kinases?
• In both yeasts only one protein kinase
• In higher eukaryotes multiple protein kinases
– Active at different stages of the cell cycle
• As with yeast different cyclins