Molar Mass! - Mrs. Luckett's Website
advertisement

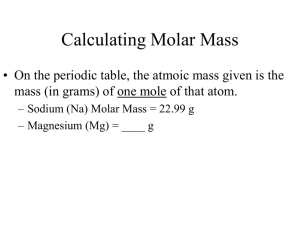
Molar Mass! Chapter Ten Chemistry Mrs. Luckett Get Out Homework Anticipation guides Molar Mass It equals how many grams are in one mole of an element or compound Examples: Element: Na molar mass is 22.990 grams Compound: NO molar mass is 30 grams Use the subscript numbers to get your total H2O = H is 1.0079 x 2 = 2.0158 O is 15.999 x 1 = 15.999 Molar mass = Calculating molar mass with parentheses When there are parentheses you have to multiply the subscript number on the outside by the subscript on the inside. Example: Mg(NO3)2 Mg = 1 x 24.305 grams = 24.305 N = (2 x 1) x14 grams = 28 O = (3 x 2) x 16 grams = 96 Total = ____ grams Practice Problems 1) What is the molar mass of the following compounds? FeO 2) Li3PO4 3) P(NO2)3 Single Step Conversions Luckett Chemistry Conversion factors You need to create two conversion factors for each equal quantity 1 mole OR molar mass 1 mole OR 22.4 L 1 mole 6.02 x 1023 particles molar mass 1 mole 22.4 L 1 mole 6.02 x 1023 particles 1 mole Example: what are the two conversion factors for the molar mass of LiNO3? Victor and Alec was here Converting moles to mass and mass to moles To convert moles to mass you have to multiply by the molar mass _____ moles x molar mass 1 mole To convert mass to moles you have to divide by the molar mass _____ grams x 1 mole molar mass Practice Problems Knowing the molar mass convert the following mole quantities to grams. 1) 0.98 moles FeO 2) 234 grams Li3PO4 3) 7.56 moles P(NO2)3 Converting Particles to Moles and Moles to Particles To convert moles to the number of particles you have to multiply by avogadro’s number _____ moles x 6.02 x 1023 particles 1 mole To convert the number of particles to moles you have to divide by avogadro’s number _____ particles x 1 mole 6.02 x 1023 particles Practice Problems Convert between moles and particles. 0.98 moles FeO 2.34 x 1025 particles Li3PO4 7.56 moles P(NO2)3 Converting Volume to Moles and Moles to Volume To convert moles to volume you have to multiply by 22.4 L _____ moles x 22.4 L 1 mole To convert volume to moles you have to divide by 22.4 L _____ Liters x 1 mole 22.4 L Practice Problems 1) Convert the following mole quantities to Liters. 2) 0.98 moles FeO 3) 234 Liters Li3PO4 4) 7.56 moles P(NO2)3