ACID BASE Worksheets
advertisement
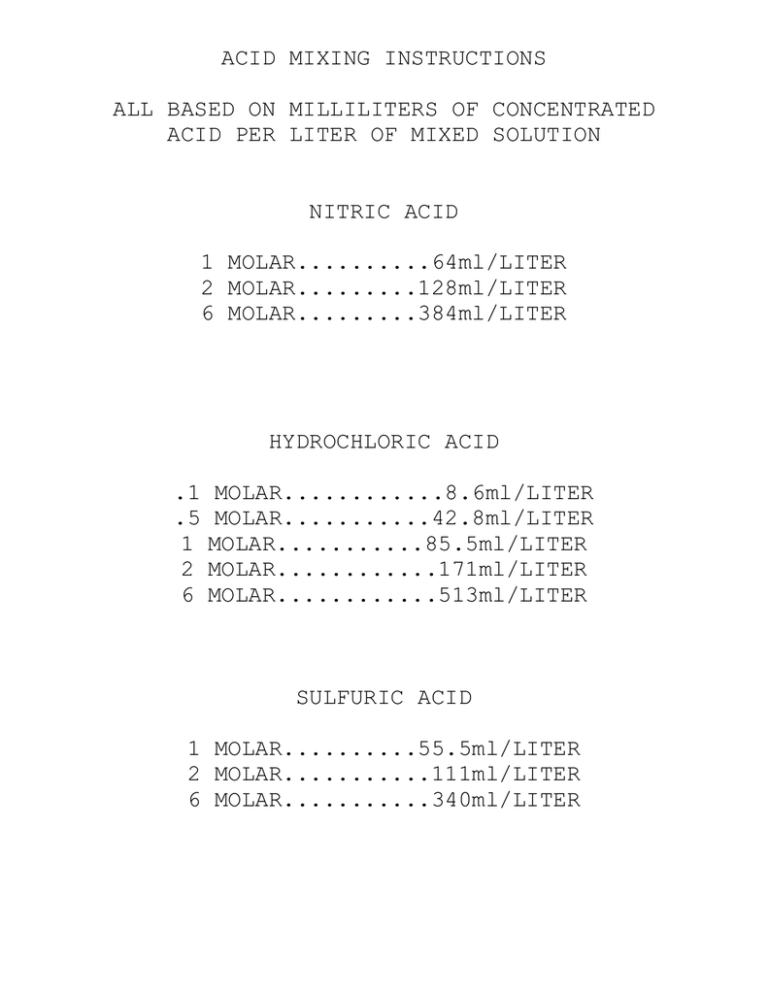
ACID MIXING INSTRUCTIONS ALL BASED ON MILLILITERS OF CONCENTRATED ACID PER LITER OF MIXED SOLUTION NITRIC ACID 1 MOLAR..........64ml/LITER 2 MOLAR.........128ml/LITER 6 MOLAR.........384ml/LITER HYDROCHLORIC ACID .1 .5 1 2 6 MOLAR............8.6ml/LITER MOLAR...........42.8ml/LITER MOLAR...........85.5ml/LITER MOLAR............171ml/LITER MOLAR............513ml/LITER SULFURIC ACID 1 MOLAR..........55.5ml/LITER 2 MOLAR...........111ml/LITER 6 MOLAR...........340ml/LITER Information Sheet: ACIDS-BASES-SALTS ACIDS-Taste sour Turn Litmus pink Corrode metals to form H2 Harmful to living tissue Neutralize all bases Conduct electricity Give off H+ in aq. Solution (Arrhenius theory) Give off protons in aq. Soln. (Bronsted-Lowry theory) Accepts 2 e-‘s forming a covalent bond (Lewis Theory) Monoprotic, Diprotic, Triprotic (all H+’s are not released at once) BASES-Taste bitter Turn litmus blue Harmful to living tissues Neutralize all acids Conduct electricity Give off OH- ions in aq. Solutions (Arrhenius) Accept protons in aq. Solution (Bronsted-Lowry) Donates 2 e-‘s forming covalent bond (Lewis Theory) SALTS-Product of an ACID-BASE neutralization Ionic compounds Contain POSITIVE ion from base; Contain NEGATIVE ion from acid Usually have high melting points Conduct electrical current in aq. solutions Molten form also conducts electricity NEUTRALIZATION OF ACIDS AND BASES (be sure to check charges and BALANCE equation) ACID + BASE --> WATER + SALT HCl + NaOH --> H20 + NaCl H+ + Cl- + Na+ + OH- --> H2O + NaCl (IONS FORMING SALT IS BASED UPON SOLUBILITY H2SO4 + Ba(OH)2 --> 2H20 + BaSO4 EQUILIBRIUM OF [H+] and [OH-} in aqueous solution. Because of neutralization, the amount of acid affects the amount of base present in aqueous solutions. MORE ACID--LESS BASE : MORE BASE--LESS ACID Kw – a mathematical relationship between [H+] and [OH-] Kw = 1E-14 Kw = [H+][OH-] Kw = 1E-14 = [H+][OH-] Example: if the [H+] = 2.5E-3 M, find the [OH-] of the solution. Kw = [H+][OH-] 1E-14 = (2.5E-3)[OH-] [OH-] = 1E-14 / 2.5E-3 = 4E-12 M Example: if the [OH-] = 3.33E-5 M, find the [H+] of the solution Kw = [H+][OH-] 1E-14 = [H+](3.33E-5) [H+] = 1E-14 / 3.33-5 = 3E-10 M pH = -(Log[H+]) pH Scale from N 0-----------------7-----------------14 Acids | Bases Strong Weak|Weak Strong Weak Acid Dissociation: [H+][A-] Ka = _________ [HA] HA H+ + A- where HA does NOT dissociate 100% but reaches equilibrium. Acid-Base Worksheet I Kw Problems Name ________________________ 1. Find the [OH-] in a .0023 M HCl solution. 2. Find the [H+] in a .000005 M NaOH solution. 3. Find the [H+] in a 8.9E-4 M Ba(OH)2 solution. 4. Find the [H+] in a 3.4e-4 M HNO3 solution. (4.35e-12) (2e-9) (5.62e-12) (3.4e-4) pH Problems pH = -(Log[H+]) pH Scale from N 0-----------------7-----------------14 Acids | Bases Strong Weal|Weak Strong Example: If the [H+] = 2.5E-5, find the phFIND THE pH. pH= -(LOG(2.5E-5)) = -(-4.6020) = 4.6020 Example: if the pH=2.3445, find the [H+]. [H+]= -(ANTILOG(-2.3445)) [H+]= 4.5E-3 PROBLEMS: 1. Find the [H+] if the pH is 12.4356. 2. FIND THE pH IF THE [H+] IS .0000411. (3.66e-13M) (4.3862) ACID-BASE I. WORKSHEET II NAME_______________________________________ WRITE THE NEUTRALIZATION EQUATIONS FOR: (BE SURE TO BALANCE) a. NITRIC ACID AND LITHIUM HYDROXIDE II. 1111 b. SULFURIC ACID AND CALCIUM HYDROXIDE 1121 c. ACETIC ACID AND AMMONIUM HYDROXIDE 1111 WHAT IS THE [H+], [OH-], AND THE pH OF A SOLUTION MADE BY ADDING 1.2 L. OF WATER TO 300 ml. OF .5 M HCL SOLUTION. [H+]=.1 M [Cl-]=.1 M [OH-]=1E-13 pH = 1 III. WHAT IS THE [H+], [OH-], AND THE pH OF A SOLUTION MADE BY ADDING 5.8 L. OF WATER TO 800 ml. OF .085 M HCL SOLUTION. [H+]=.0103 M [Cl-]=.0103 M [OH-]= 9.71e-13 pH = 1.9872 IV. IF 15 GRAMS OF BARIUM HYDROXIDE ARE DISSOLVED INTO WATER TO MAKE 176 L. OF SOLUTION, FIND THE [Ba+2], [OH-], [H+] AND THE pH OF THE SOLN. [Ba+2]=.000498 [OH-]=.000999 [H+]=1e-11 pH=11.0000 Acid-Base Worksheet III I. II. Name______________________ Per_____ IF THE pH OF A NITRIC ACID SOLUTION IS 3.3040, FIND THE [H+], [NO3-] AND THE [OH-]. [H+]=.000497 [NO3-]=.000497 [OH-]=2e-11 IF 200 ml OF .35 M HNO3 IS MIXED WITH 400 ml OF .2 M RbOH CALCULATE WHICH CHEMICAL IS IN EXCESS, THE [H+], [OH-], [Rb+], AND THE pH OF THE RESULTING SOLUTION. EXCESS=.01 MOLES OH[OH-]=.0167 M [H+]= 5.98e-13 M pH= 12.2229 III. YOU ARE TITRATING 30 ml OF UNKNOWN ACID WITH .35 M KOH. THE SOLUTION TURNS PINK AFTER 45.6 ml OF THE BASE IS ADDED. CALCULATE THE [H+], [OH-] AND THE pH OF THE ACID SOLUTION. pH=.2738 [H+]=.533 M [OH-]=1.87E-14 IV. IF 300 ml OF .45 M HNO3 IS MIXED WITH 400 ml OF .2 M Ca(OH)2 CALCULATE WHICH CHEMICAL IS IN EXCESS, THE [H+], [OH-], [Ca+2], AND THE pH OF THE RESULTING SOLUTION. EXCESS=.025 MOLES OH[OH-]=.0357 M [H+]=2.8E-13 M pH= 12.5527 V. YOU ARE TITRATING 25 ml OF UNKNOWN ACID WITH 1.5 M KOH. THE SOLUTION TURNS PINK AFTER 8.2 ml OF THE BASE IS ADDED. CALCULATE THE [H+] AND THE pH OF THE ACID SOLUTION. [H+]=.492M pH=.3080 Acid-Base Worksheet IV I. II. III. Name _______________________ Per _______ (Honors Only) 7.5 GRAMS OF THE ACID HA IS MIXED WITH WATER TO 2.5 LITERS. HA DISSOCIATES INTO H+ IONS AND A- IONS. THE MOLAR MASS OF HA IS 100 GRAMS/MOLE. FIND THE [H+], [A-], [HA], [OH-], & pH. Ka=9.5E-9 (weak acid dissociation) [HA]=2.99E-2 [H+]=1.69E-5 [OH-]=5.92E-10 WRITE THE NEUTRALIZATION EQUATIONS FOR: (BE SURE TO BALANCE) a. PHOSPHORIC ACID AND LITHIUM HYDROXIDE 1331 b. SULFURIC ACID AND ALUMINUM HYDROXIDE 3261 c. ACETIC ACID AND BARIUM HYDROXIDE 2121 HOW MANY HYDROGEN IONS ARE IN 50 ml OF .025 M HBr SOLUTION? 7.53e20 ions IV. WHAT IS THE MOLARITY OF HYDROGEN IONS IN AN ACID SOLUTION WHOSE pH IS LISTED AS 1.3342? [H+]=4.63E-2M V. IF 150 ml OF .035 M HI ARE MIXED WITH 150 ml OF .015 M Ca(OH)2, FIND ALL ION CONCENTRATIONS IN RESULTING SOLUTION. ASSUME BOTH CHEMICALS ARE STRONG ELECTROLYTES. [I-]=1.75E-2 [Ca++]=7.5E-3 [H+]=2.5E-3 [OH-]=4E-12 pH=2.6021 VI. a.) WHAT IS THE MOLARITY OF AN UNKNOWN ACID IF 35 ml OF IT ARE TITRATED TO EQUIVILENCE BY 17.3 ml OF .15 M NaOH. 7.41E-2M b.) HOW MANY GRAMS OF SOLID SODIUM HYDROXIDE WILL BE NEEDED TO MAKE 100 ml OF A BASE SOLUTION THAT IS AS STRONG AS THE UNKNOWN ACID? 2.97E-1g VII. (Honors only)A 25 GRAM SAMPLE OF BENZOIC ACID (C6H5COOH)IS DISSOLVED IN WATER TO 1200 ml. FIND THE [SPECIES] AT EQUILIBRIUM. Ka=6.5E-6 FIND THE pH ALSO. X=1.05E-3M [C6H5COOH] = 1.699e-3M