Nuclear Reactions:
advertisement

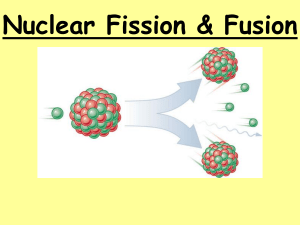
Unit 8 Section 2: Nuclear Reactions An Introduction to Nuclear Fission and Fusion Objectives: • Explain that nuclear reactions deal with interactions between the nuclei of atoms and chemical reactions occur when electrons interact. • Describe the relationship between matter and energy. • Explain and differentiate between nuclear fission and nuclear fusion. • Understand that both fission and fusion processes deal with matter and energy. Nuclear Energy vs. Chemical Energy • What is the difference between nuclear and chemical energy? • Chemical Energy: Energy that is released during chemical reactions. Chemical energy involves electrons. • Nuclear Energy: Energy that is released from the atomic nucleus. This involves much larger energies than chemical reactions. Matter and Energy • Recall the law of conservation of energy: • Energy cannot be created or destroyed. • New concept: • Matter and energy are 2 forms of the same thing Einstein’s Famous Equation:E = mc2 • Matter can be changed into Energy • Einstein’s formula tell us how the change occurs • In E = mc2, • E = Energy • m = Mass • c = Speed of Light (Universal Constant) E= 2 mc • The equation can be read as follows: • Energy (E) is equal to Mass (m) multiplied by the Speed of Light (c) squared • This tells us that a small amount of mass can be converted into a very large amount of energy because the speed of light (c) is an extremely large number • c = 3.0 x 108 m/s Fission: What is it? • Fission: • The process of splitting an atomic nucleus into fission fragments. • Fission fragments(products): • In the form of smaller atomic nuclei and neutrons • Releases radiation Fission (cont’d) • LARGE amounts of energy are produced by the fission process • Fission involves heavy atoms with a lot of protons and neutrons (nucleons) • How and why does fission happen? • How: the nuclei of the heavy atoms are hit by neutrons; this starts the fission process. • Why: fission occurs because of the electrostatic repulsion created by large numbers of protons within the nuclei of heavy atoms. Example of Fission Reaction • Start with: • U-235 + 1 neutron • Product: • 2 neutrons + Kr-92 + Ba-142 + Energy Explanation of Fission Reaction • Explanation: • A neutron hits an atom of U-235. • It absorbs the neutron and becomes U-236 which is unstable. • Fission occurs and more neutrons are released in the reaction. • The stray neutrons strike other U-235 atoms to start a nuclear chain reaction. The Fission Process Fission (cont’d) • The fission process is NATURAL • There was a natural uranium reactor in West Africa over 2 billion years ago. • Why is fission useful? • It generates a large amount of energy and this heat can be captured by nuclear power plants to produce electricity. What elements are good for fission? • Uranium 235 (U-235) • Uranium 238 (U-238) • Elements with an atomic number higher than 92 do not exist in nature; they are also good for the fission process. Fusion: What is it? • Fusion: • A nuclear reaction in which two light nuclei combine or fuse to make a larger, heavier nucleus. • This process generates LOTS of energy (Remember E = mc2). • In order for fusion to begin, a large amount of energy is needed to overcome the electrical charges of the nuclei and fuse them together. Fusion Facts • Fusion reactions DO NOT occur naturally on our planet, but nuclear fusion is what powers the stars. • The sun fuses hydrogen atoms to produce helium, subatomic particles, and a large amount of energy. Diagram of Nuclear Fusion Review • Mass and energy are two forms of the same thing; neither can be created or destroyed, but mass can be converted into energy (E = mc2). • Fission is a nuclear reaction in which: • A heavy atomic nucleus is split into lighter atomic nuclei. • Fusion is a nuclear reaction in which: • 2 light nuclei are combined into a single, heavier atomic nucleus. Quiz Question #1 • Which nuclear process produces large amounts of energy? A. Fission B. Fusion C. Both fission and fusion D. Neither fission or fusion Quiz Question #1 • Which nuclear process produces large amounts of energy? A. Fission B. Fusion C. Both fission and fusion D. Neither fission or fusion Quiz Question #2 • Fission is the process that __________ atomic nuclei. A. combines B. burns up C. stores D. splits Quiz Question #2 • Fission is the process that __________ atomic nuclei. A. combines B. burns up C. stores D. splits Quiz Question #3 • Mass may be converted into energy. A. True B. False Quiz Question #3 • Mass may be converted into energy. A. True B. False Quiz Question #4 • The fission process requires heavy atomic nuclei. A. True B. False Quiz Question #4 • The fission process requires heavy atomic nuclei. A. True B. False Quiz Question #5 • Name the nuclear reaction that occurs within the sun. Quiz Question #5 • Name the nuclear reaction that occurs within the sun. • Nuclear Fusion Quiz Question #6 • Fission is a natural process that occurs on the planet earth. A. True B. False Quiz Question #6 • Fission is a natural process that occurs on the planet earth. A. True B. False Quiz Question #7 • Explain this equation: • E = mc2 Quiz Question #7 • Explain this equation: • E = mc2 • Energy is equal to mass times the speed of light squared. https://www.youtube.com/watch? v=KWAsz59F8gA