Acids
pH below 7
turns litmus paper red
taste sour
reacts with metals to produce H2(g)
generally starts with a hydrogen ion
[H+] > [OH-]
HCl
2
Bases
pH greater than 7
turns litmus paper blue
taste bitter
feel slippery
generally contains a hydroxide ion
[H+] < [OH-]
NaOH
3
Both Acids and Bases
an electrolyte
4
Acidic, Basic, and Neutral
Solutions
Type of
Solution
pH Ranges
[H+] versus [OH-]
Example
Orange Juice
Battery Acid
Your Stomach
Acidic
Below 7
[H+] > [OH-]
Neutral
Equals
EXACTLY 7
[H+] = [OH-]
Basic
Above 7
[H+] < [OH-]
Distilled
Water
Bleach
Sea Water
Blood
5
Indicators
Indicators are compounds that have one
color in acidic solutions and another in
basic.
Litmus Paper
Copyright © 2007 Pearson Benjamin Cummings. All rights reserved.
Copyright © 2007 Pearson Benjamin Cummings. All rights reserved.
BINARY ACIDS
HBr (aq)
Hydrobromic
Acid
Naming
Ternary
Acids
TERNARY
ACIDS
POLYATOMIC PURE
IONS
FORMS
2-
H2SO4
SO32-
H2SO3
SO4
TERNARY ACIDS
H2SO4(aq)
Sulfuric acid
H2SO3(aq)
Sulfurous Acid
Naming Bases
Use the same rules as for
ions (name the cation, then name the anion)
NaOH
Ca(OH)2
KOH
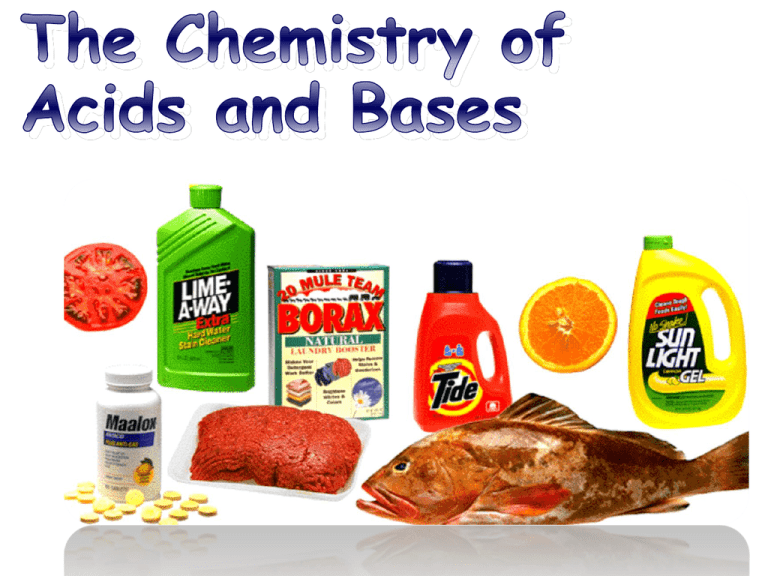
Some Common Acids and Bases
and their Household Uses.
What are Acids and Bases?
There are two common definitions to
describe acids and bases:
1.
2.
Arrhenius acids and bases
Bronsted-Lowry acids and bases
These are basically the same although they
state different things.
Definitions for Acids & Bases
Arrhenius
Brønsted-Lowry
Definition for
Acids
a proton producer in an
aqueous solution
a proton donor
Definition for
Bases
a hydroxide producer in
an aqueous solution
a proton acceptor
Acid – HCl
Acid – HCl
Base - NaOH
Base – NH3
Key Examples
H+ = proton
Arrhenius Acids and Bases
Definitions
1. Arrhenius Acid
acids in water produce hydronium ions, (H3O+, H+)
HNO3(aq) H+(aq) + NO32. Arrhenius Base
bases in water produce hydroxide ions, (OH-)
KOH(s) K+(aq) + OH-(aq)
Bronsted-Lowry Definitions
• Acids are proton (H+) donors.
• Bases are proton (H+) acceptors.
HCl + H2O
acid
–
Cl
+
+
H3O
base
conjugate base
conjugate acid
Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem
Bronsted-Lowry
Come in Pairs
General equation
HA(aq) + H2O(l)
A-(aq) + H3O+(aq)
Acid + Base
Conjugate base + Conjugate acid
This is an equilibrium.
B(aq) + H2O(l)
Base + Acid
BH+(aq) + OH-(aq)
Conjugate acid +Conjugate base
This is an equilibrium.
What to Focus On?
Arrhenius was the most restrictive definition. This definition
required:
the solutions to be aqueous and
a base to contain a hydroxide (OH-) ion.
Bronsted-Lowry’s definition is the most commonly used. It is helpful
to remember:
acids tend to “lose“ an H+ ion, while
bases tend to “gain“ an H+ ion.
Under this definition, ammonia (NH3) is considered a base even though
it is NOT an Arrhenius base.
Examples
HCl(aq) + KOH(s) KCl(aq) + H2O(l)
3 Ca(OH)2(aq) + 2 H3PO4(aq) Ca3(PO4)2(s) + H2O(l)
acid
base
acid
base
F-(aq) + H2O(l) HF(aq) + OH-(aq)
base
conjugate
acid
acid
conjugate
base
HCO3-(aq) + H2O(l) CO32-(aq) + H3O+(aq)
NH4+(aq) + CO32-(aq) NH3(aq) + HCO3-(aq)
acid
acid
base
base
conjugate
base
conjugate
base
conjugate
acid
conjugate
acid
Remember Electrolytes?
Ionic
Covalent
C6H12O6
NaCl
ClNa+
Na+
Cl-
C6H12O6
C6H12O6
Acids and bases are both strong or weak electrolytes
(conduct electricity)
• Electrolytes = dissociate (break apart into ions) when dissolved
• Strong = completely Weak = partially
Non = not at all
Weak
Strong
HC2H3
O2
H-Cl
C2H3O21
Cl
-
H
+
H
+
C2H3O21-
H
-
H
+
+
Cl
-
Only a few Ions
Lots of Ions
WORD DESCRIPTION
Completely
Notice breaks
that all
apart into its ions
of the ions are
Are good conductors
separated or
of electricity
Willdissociated.
produce a bright
light bulb
Examples of Acids and
Bases that are Strong
Electrolytes
Strong Acids
H2SO4
HCl
Strong Bases
NaOH
Ba(OH)2
23
WORD DESCRIPTION
Notice
that only
Partially breaks apart
some
the ions
into itsof
ions
are
Are separated
poor conductors
of
or
electricity
dissociated.
Will produce a dim light
bulb
Examples of Acids and
Bases that are Weak
Electrolytes
Weak Acid
HC2H3O2 (Vinegar)
Weak Base
NH3 (Ammonia)
24
Strong electrolytes make strong
acids and bases
Strong Bases
Strong Acids
The hydroxides of the
Group I and Group II
HCl - hydrochloric acid
HBr - hydrobromic acid
HI - hydroiodic acid
HNO3 - nitric acid
H2SO4 - sulfuric acid
HClO4 - perchloric acid
LiOH - lithium hydroxide
NaOH - sodium hydroxide
KOH - potassium hydroxide
*Ca(OH)2 - calcium hydroxide
*Sr(OH)2 - strontium hydroxide
*Ba(OH)2 - barium hydroxide
25
pH Concept
pH Scale
Pouvoir hydrogéne (hydrogen power)
Is a scale to measure the acidity of a
sample, Range: 0 -14
1
Highly acidic
Acids 0-7
7
neutral
Neutral = 7.0
14
Very basic
(not acidic)
Bases 7-14
Relationships between pH, [H+],
and [OH-]
As pH increases…
The [H+] (increases or decreases).
The [OH-] (increases or decreases).
The solution becomes more (acidic or basic).
Relationships between pH,
[H+], and [OH-]
What happens as pH decreases?
As pH decreases…
The [H+] (increases or decreases).
The [OH-] (increases or decreases).
The solution becomes more (acidic or basic).
The pH Scale
The value of pH is
unitless.
Solutions with a pH less
than 7 are acidic and
solutions greater than
7 are basic.
If a solution is equal to
7 it is neutral.
Here is a typical pH
scale.
pH of Common Substances
pH ispH
a Logarithmic
Scale
is a Logarithmic Scale
Logarithm –The number of times a
base must be multiplied by itself to
reach a given number
# of multiples
x log b y
Base
# you’re trying to reach
pH Calculations
Given
Solving for Formula to Use
[H+]
pH
pH = - log[H+]
[OH-]
pOH
pOH = - log[OH-]
[H+] is the concentration of H+ ions, in mol/L.
Logarithms
Use your calculator!
If you have a log button, you’re all set.
Each calculator can have its
own method for entering logs.
1.44939 E -2
If you don’t know what to do
your
calculator manual
should give examples.
cos tan
CE
ln
7
8
9
/
log
4
5
6
x
1/x
1
2
3
-
x2
EE
0
.
+
9 - 43
Logarithms
If your calculator has a ln button 1.44939 E -2
• Don’t use it.
• Its for taking natural logs.
• This is different than base 10.
cos tan
CE
ln
7
8
9
/
log
4
5
6
x
1/x
1
2
3
-
x2
EE
0
.
+
9 - 44
Calculating pH
If [H+] is written in scientific notation and
has a coefficient of 1, then the pH of the
solution equals the absolute value of the
exponent
Ex. 1.0 x 10-4 M
pH = 4.0
Calculating pH
Problem 1:
If [H+] = 3.40 x 10-5 M, what is the pH?
Given
Unknown
Equation
[H+] = 3.40 x 10-5 M
pH
pH = - log[H+]
Solve:
pH = -log (3.40 x 10-5)
pH = 4.47
Calculating pH
Problem 2:
If [H+] = 1 X 10-10, what is the pH?
Given
Unknown
Equation
[H+] = 1 X 10-10 pH
pH = - log[H+]
Solve:
pH = - log 1 X 10-10
pH = - (- 10)
pH = 10
Calculating pH
Problem 3:
If [H+] = 1.8 X 10-5, what is the pH?
Given
Unknown
Equation
[H+] = 1.8 X 10-5 pH
pH = - log[H+]
Solve:
pH = - log 1.8 X 10-5
pH = - (- 4.74)
pH = 4.74
Calculating pOH
Problem 1:
If [OH-] = 2.30 x 10-12 M, what is the pOH?
Given
Unknown
Equation
[OH-] = 2.30 x 10-12 M
pOH
Solve:
pOH = -log (2.3 x 10-12)
pOH = 11.6
pOH = - log[OH-]
Calculating pOH
If [OH-] is written in scientific notation and
has a coefficient of 1, then the pOH of the
solution equals the absolute value of the
exponent
Problem 2:
If [OH-] = 1.0 x 10-9 M, what is the pH?
pOH = 9.0
WHAT’S IN A
distilled
GLASS OF
WATER?
Distilled H2O at the Molecular
LevelWhat’s in a glass of distilled water?
• Water Molecules (H2O)
• Hydronium Ions (H3O+)
• Hydroxide Ions (OH-)
What’s happens in the glass of
water?
H2O + H2O ⇆ H3O+ + OHThis is called the self-ionization of
water.
Water
Water ionizes- falls apart into ions.
H2O H+ + OH-.
Only a small amount.
[H+ ] = [OH-] = 1 x 10-7M
A neutral solution.
In water Kw = [H+ ] x [OH-] = 1 x 10-14
Kw is called the ion product constant.
pH
+ pOH = 14
Amphoteric
a molecule or ion
that can react as
an acid as well as
a base
Ex: H2O, NH3
Calculating pOH from pH
Problem 1:
If the pH is 3.25, what is the pOH?
Given
pH = 3.25
Unknown
pOH ?
Substitute and solve :
3.25 + pOH = 14
3.25 + (- 3.25) +pOH = 14 (- 3.25)
pOH = 10.8
Equation
pH + pOH = 14
Calculating pH from pOH
Problem 2:
What is the pH of a solution if [OH-] = 4.0 x 10-11 M?
Given
[OH-] = 4.0 x 10-11 M
Unknown
pH?
Step 1: Find pOH
pOH = -log [OH]
pOH= -log[4.0 x 10-11 ] = 10.4
Step 2: Calculate pH
pH + pOH= 14; pH = 14 – 10.4
pH = 3.6
Equation
pH + pOH = 14
Looking at the Math
Given
Solving for Formula to Use
pH
[H+]
pOH
[OH-]
[ H ] 10
pH
[OH ] 10
pOH
Calculating [H+] from pH
If the pH of Coke is 3.12, [H+] = ???
Known
[
pH = 3.12
Unknown
[H+] ?
Analysis
[H+] = 10 -pH
Substitute and solve :
[H+] = 10-3.12 = 7.6 x 10-4 M
*** to find antilog on your calculator, look for “Shift” or “2nd function”
and then the log button
Calculating [H+] from pH
The pH of an unknown solution is 6.00.
What is its [H+]?
Known
pH = 6.00
Unknown
[H+] ?
Substitute and solve :
[H+] = 1x 10 -6 M
Analysis
[H+] = 10 -pH
Calculating [H+] from pH
A solution has a pH of 8.5. What is the Molarity
of hydrogen ions in the solution?
Known
pH = 8.5
Unknown
[H+] ?
Substitute and solve :
[H+] = 10-8.5
3.16 X 10-9 M
Analysis
[H+] = 10 -pH
Acid-Base Reactions or
Neutralization Reactions
acid + base water + salt
1.
2.
3.
HBr(aq) + NaOH(aq) H2O + NaBr
H2SO4(aq) +
KOH(aq) H2O + K2SO4
H3PO4(aq) + Ba(OH)2(aq) H2O + Ba3(PO4)2
* Double replacement reactions





