Draw: methyl cis-3-ethylcyclobutanecarboxylate 8. Name: 8. 9
advertisement

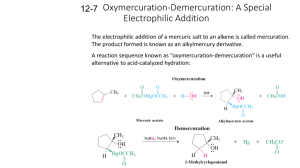
6.Draw: methyl cis-3-ethylcyclobutanecarboxylate 8.Name: ____________________________________________ 8. 9. Name: _____________________________________________ 10.Name: ___________________________________________ 11.Name: ______________________________________________ Questions 15 -17: Consider the data in the Table below to answer the following question(s): 16. Acidities of Substituted Benzoic and Acetic Acids pKas at 25C YC6H4COOH Y meta YCH2COOH H 4.75 4.19 CN 2.47 3.64 OCH3 3.57 4.09 Which of the acids in the table above has the strongest conjugate base? ______________________________ para 4.19 3.55 4.46 19 Explain the differences in acidity between p-methoxybenzoic acid and m-methoxybenzoic acid. When CO2 is bubbled through an ether solution of benzylmagnesium bromide, and the resulting mixture is acidified, phenylacetic acid is produced. Any unreacted benzylmagnesium bromide is converted to toluene in the acidification step. a. b. c. d. 20. Write the complete reaction sequence for the process described above. 21. How could you separate phenylacetic acid from toluene? 22. This reaction is described as a _____ process. carbonylation carboxylation Carbaniolation cationation Circle one: a b c d Give the major organic product(s) for each of the following reactions or sequences of reactions. Show all relevant stereochemistry. 26. Question 28: What is the order of decreasing reactivity towards nucleophilic acyl substitution for the carboxylic acid derivatives? (most reactive first) 28. a. b. c. d. I, II, III, IV I, III, IV, II II, IV, III, I II, I, III, IV Circle one: a b c d Questions 31 and 32: Consider the reaction below to answer the following question(s): Acid halides react with diazomethane to yield diazoketones. Excess diazomethane is used to prevent the HCl produced in the reaction from reacting with the diazoketone. 31. Diazomethane is an example of a dipolar molecule; a molecule which is neutral overall but has charges on individual atoms. One resonance form of diazomethane is drawn below. Draw the Lewis structure of the other resonance form of diazomethane. Be sure to include all formal charges. 32. The intermediate structures for the mechanism for the reaction of propanyl chloride with diazomethane are provide below. Show all electron flow with arrows on these structures. 33. Methyl butanoate has been isolated from pineapple oil and can be prepared by the Fischer esterification reaction shown below. Write the complete stepwise mechanism for this reaction. Show all electron flow with arrows and include all intermediate structures. 34. The purpose of the acid catalyst in the hydrolysis of an amide is: a. b. c. d. to enhance the nucleophilicity of the water molecule to enhance the electrophilicity of the amide carbonyl carbon to enhance the electrophilicity of the water molecule to shift the equilibrium of the reaction Circle one: a b c d Questions 35 - 37: Consider the information below to answer the following question(s). The reaction of a carboxylic acid with an alcohol in the presence of acid is termed Fischer esterification. 35. The nucleophile in this reaction is _____. 36. Compound C functions as _____ in this reaction. a. b. c. d. a base scavenger a solvent a catalyst a neutralizer Circle one: a b c d