Intensive General Chemistry
advertisement

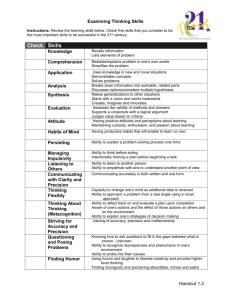
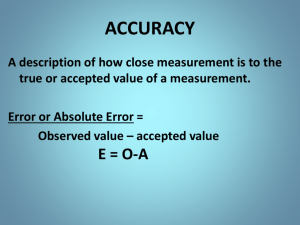
Intensive General Chemistry Uncertainty Analysis Wet Techniques Luis Avila Isabelle Vu Trieu avila@chem.columbia.edu ilv2@columbia.edu Introduction • Measurement and Uncertainty • Qualitative Analysis • What is in the unknown? • Quantitative Analysis • How much of it is in the unknown? Uncertainty in Measurement • Measurements always involve a comparison • The comparison always involve some uncertainty Length of the beetle’s body? -between 0 and 2 in -between 1 and 2 in -between 1.5 and 1.6 in -between 1.54 and 1.56 in -between 1.543 and 1.548 in Convention: Read 1/10 of the distance between the smallest scale division Significant Figures • Definition: – all digits up to and including the first uncertain digit • Ex: Beetle’s length is 1.55 in (3 sig fig) 4.0 cm (2 sig fig) 0.04 m (1 sig fig) – The more significant figures, the more reproducible a measurement is (ex: ∏) – Counts and defines numbers are exact - They have no uncertain digits! Counting significant figures in a series of measurements • Compute the average • Identify the first uncertain digit • Round the average so that the last digit is the first uncertain digit Ex: Beetle’s length » Measurement 1: 3.98 cm » Measurement 2: 4.01 cm » Measurement 3: 4.00 cm AVERAGE = 4.00 cm or 4.00 x 10-2 m Precision of Calculated Results • calculated results are never more reliable than the measurements they are built from • multistep calculation: never round intermediate results! • Sums and differences: round result to the same number of fraction digits as the poorest measurement Ex: 4.01+ 22.2222 = 26.23 • Products and quotients: round result to the same number of significant figures as the poorest measurement Ex: 4.01 x 22.2222 = 89.1 Precision versus Accuracy good precision & good accuracy poor precision but good accuracy good precision but poor accuracy poor precision & poor accuracy Precision versus Accuracy Random Errors versus Systematic Errors • Precision – Reproducibility – Check by repeating measurements – Poor precision results from poor technique • Random Errors – Random sign – Varying magnitude • Accuracy – Correctness – Check by using a different method – Poor accuracy results from procedurial or equipment defects • Systematic Errors – Reproducible sign – Reproducible magnitude Estimating Precision Standard Deviation the ith value sample mean standard deviation total number of measurements Expressing Experimental Error • Absolute error = Magnitude of the random error Ex: Beetle’s length = 4.00 ± 0.02 cm • Relative error = Ratio of the absolute error to the measurement Ex: 0.02/4.00 = .005 = 5% Beetle’s length = 4.00 ± 5% cm All your final experimental results must be reported with absolute error. Propagation of Errors Result obtained by adding or subtracting experimental quantities absolute error = sum of the absolute errors in the exp quantities Result obtained by multiplying or dividing exp quantities relative error = sum of the relative errors in the exp quantities Absolute error = Relative error x Measurement 4.00 ± 0.02 cm Perimeter? 12.00 ± 0.08 cm Area? cm2 8.00 ± (1% + 0.5%) 8.0 ± 0.1 cm2 Propagation of Errors Result obtained by multiplying or dividing an exp qty by a constant Absolute error = same constant x absolute error in the exp quantity Logarithmic expression Relative error = 0.434 x relative error in the exp quantity Average Absolute error = greatest absolute error in exp quantities being averaged Only absolute errors can be used for final results Wet Techniques • Experiments: – – – – Calibrating Glassware Preparation of standards Titration Qualitative Analysis for Cations • Collaborative/Cooperative work necessary! Calibrating Glassware • Volumetric glassware: – “to contain” (TC) – “to deliver” (TD) • Objectives: – Estimate precision of volumetric glassware – Compare with manufacturer’s uncertainty Gravimetric Calibration • Determine: – Mass of water in the Measured volume – Temperature of water • Calculate: – Volume of water (using the density of water) • Compare: – Calculated and Measured Vwater. Qualitative Analysis for Cations • Objectives – Design a Cation Analysis Scheme – Identify and Separate Cations in a mixture Cation Analysis Scheme Volumetry • Objectives – Prepare solutions of known concentration from primary and non-primary standards – Perform titrations Pre-lab questions • E2 - 6: Retrieve the MSDS of KHP and NaOH. Calculate the mass of NaOH and KHP needed in order to prepare the solutions. • E2 - 12: Sketch an alternative analysis scheme starting with precipitation with NaOH instead of HCl.