Conventional Therapy Triple Therapy
advertisement

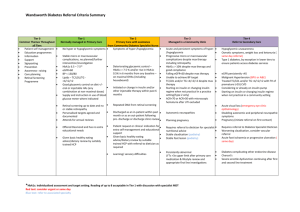
Journal Club Writing Group for the DCCT/EDIC Research Group, Orchard TJ, Nathan DM, Zinman B, Cleary P, Brillon D, Backlund JY, Lachin JM. Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA. 2015 Jan 6;313(1):45-53. Abdul-Ghani MA1, Puckett C, Triplitt C, Maggs D, Adams J, Cersosimo E, DeFronzo RA. Initial combination therapy with metformin, pioglitazone and exenatide is more effective than sequential add-on therapy in subjects with new-onset diabetes. Results from the Efficacy and Durability of Initial Combination Therapy for Type 2 Diabetes (EDICT): a randomized trial. Diabetes Obes Metab. 2015 Mar;17(3):268-75. 2015年2月19日 8:30-8:55 8階 医局 埼玉医科大学 総合医療センター 内分泌・糖尿病内科 Department of Endocrinology and Diabetes, Saitama Medical Center, Saitama Medical University 松田 昌文 Matsuda, Masafumi 1型糖尿病 DCCT/EDIC -42% (P=0.02) 0.12 心 血 管 イ ベ ン ト の 累 積 発 生 率 0.10 従来療法群(N=589) 0.08 0.06 A1C 7.4% vs 9.1% A1C 8.0% vs 8.2% 0.04 0.02 強化療法群(N=593) 0.00 0 1 2 3 4 5 No. at Risk 強化療法群 従来療法群 705 714 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 観察期間(年) 683 688 629 618 113 92 N Engl J Med 2005; 353: 2643-53. Diabetic retinopathy cumulative incidence Secondary prevention 76% conventional 54% Incidence Incidence Primary prevention conventional intensive intensive years years conventional conventional intensive intensive 4 FIG. 2. Estimated cumulative incidence of further 3-step progression of retinopathy from DCCT closeout, by DCCT treatment group, through EDIC year 4, for adolescents (A) and for adults (B); through EDIC year 10, for adolescents (C) and for adults (D). Subjects with prior scatter photocoagulation during DCCT (7 adolescents and 29 adults) were excluded from analyses. Based on Weibull regression models adjusted for the level of retinopathy at the end of the DCCT, primary vs. secondary cohort, the A1C value on entry to the DCCT, and diabetes duration at DCCT baseline. Hazard reduction was for intensive therapy compared with conventional therapy. Diabetes 59:1244–1253, 2010 Figure 1. Cumulative Incidence of an Impaired Glomerular Filtration Rate, According to Treatment Group. An impaired glomerular filtration rate (GFR) was defined as a sustained estimated GFR of less than 60 ml per minute per 1.73 m2 of body-surface area. The cumulative incidence of an impaired GFR is shown according to the group to which the participants had been randomly assigned in the Diabetes Control and Complications Trial, with death accounted for as a competing risk. The hazard ratio and P value were calculated with the use of a Cox proportional-hazards model with a robust estimate of confidence limits according to the method of Lin and Wei16 and the robust Wald test. de Boer IH, Sun W, Cleary PA, Lachin JM, Molitch ME, Steffes MWZinman B, ; DCCT/EDIC Research Group: Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med. 2011; 365: 2366-76 University of Pittsburgh, Pittsburgh, Pennsylvania (Orchard); Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts (Nathan); Lunefeld Tanenbaum Research Institute, Mount Sinai Hospital, University of Toronto, Toronto, Ontario, Canada (Zinman); The George Washington University Biostatistics Center, Rockville, Maryland (Cleary, Backlund, Lachin); Cornell University Medical Center, New York, New York (Brillon). JAMA. 2015;313(1):45-53. Importance Whether mortality in type 1 diabetes mellitus is affected following intensive glycemic therapy has not been established. Objective To determine whether mortality differed between the original intensive and conventional treatment groups in the longterm follow-up of the Diabetes Control and Complications Trial (DCCT) cohort. Design, Setting, and Participants After the DCCT (1983-1993) ended, participants were followed up in a multisite (27 US and Canadian academic clinical centers) observational study (Epidemiology of Diabetes Control and Complications [EDIC]) until December 31, 2012. Participants were 1441 healthy volunteers with diabetes mellitus who, at baseline, were 13 to 39 years of age with 1 to 15 years of diabetes duration and no or early microvascular complications, and without hypertension, preexisting cardiovascular disease, or other potentially life-threatening disease. Interventions and Exposures During the clinical trial, participants were randomly assigned to receive intensive therapy (n = 711) aimed at achieving glycemia as close to the nondiabetic range as safely possible, or conventional therapy (n = 730) with the goal of avoiding symptomatic hypoglycemia and hyperglycemia. At the end of the DCCT, after a mean of 6.5 years, intensive therapy was taught and recommended to all participants and diabetes care was returned to personal physicians. Main Outcomes and Measures Total and cause-specific mortality was assessed through annual contact with family and friends and through records over 27 years’ mean follow-up. Results Vital status was ascertained for 1429 (99.2%) participants. There were 107 deaths, 64 in the conventional and 43 in the intensive group. The absolute risk difference was −109 per 100 000 patient-years (95% CI, −218 to −1), with lower all-cause mortality risk in the intensive therapy group (hazard ratio [HR] = 0.67 [95% CI, 0.46-0.99]; P = .045). Primary causes of death were cardiovascular disease (24 deaths; 22.4%), cancer (21 deaths; 19.6%), acute diabetes complications (19 deaths; 17.8%), and accidents or suicide (18 deaths; 16.8%). Higher levels of glycated hemoglobin (HbA1c) were associated with all-cause mortality (HR = 1.56 [95% CI, 1.35-1.81 per 10% relative increase in HbA1c]; P < .001), as well as the development of albuminuria (HR = 2.20 [95% CI, 1.46-3.31]; P < .001). Conclusions and Relevance After a mean of 27 years’ follow-up of patients with type 1 diabetes, 6.5 years of initial intensive diabetes therapy was associated with a modestly lower all-cause mortality rate when compared with conventional therapy. Trial Registration clinicaltrials.gov Identifiers: NCT00360815 and NCT00360893 Message 1型糖尿病(DM)患者の死亡率が血糖コントロー ルの影響を受けるかを、血糖コントロールと合 併症に関する大規模臨床試験(DCCT試験)参加 者1441人の長期追跡(EDIC試験)から検討。平 均27年の追跡の結果、全死因死亡リスクは標準 療法に対して強化療法群で低く(ハザード比 0.67)、絶対リスク差は10万人年当たり-109 だった。 September, 8, 2008 in Rome at EASD meeting BETA CELL FAILURE IN T2DM: CAN IT BE PREVENTED? Ralph A. DeFronzo, M.D. Professor of Medicine Division of Diabetes UTHSCSA ADA ALGORITHM Lifestyle + Metformin HbA1c > 7.0% Add Basal Insulin Add Sulfonylurea Add Glitazone Intensify Insulin Add Glitazone or Basal Insulin Add SU or Basal Insulin PATHOPHYSIOLOGIC BASED (DEFRONZO) ALGORITHM TRIPLE COMBINATION: Pioglitazone + Metformin + Exenatide HbA1c < 6.0% 1.Diabetes Division, University of Texas Health Science Center at San Antonio, San Antonio, TX, USA 2.GI Dynamics, Lexington, MA, USA Aim To test our hypothesis that initiating therapy with a combination of agents known to improve insulin secretion and insulin sensitivity in subjects with new-onset diabetes would produce greater, more durable reduction in glycated haemoglobin (HbA1c) levels, while avoiding hypoglycaemia and weight gain, compared with sequential addition of agents that lower plasma glucose but do not correct established pathophysiological abnormalities. Methods Drug-naïve, recently diagnosed subjects with type 2 diabetes mellitus (T2DM) were randomized in an open-fashion design in a single-centre study to metformin/pioglitazone/exenatide (triple therapy; n = 106) or an escalating dose of metformin followed by sequential addition of sulfonylurea and glargine insulin (conventional therapy; n = 115) to maintain HbA1c levels at <6.5% for 2 years. Study Design Eligible participants were consecutively randomized based on age, sex, BMI, diabetes duration and HbA1c level to receive either initial triple combination therapy with metformin/pioglitazone/exenatide or metformin with sequential addition of glipizide and then basal insulin glargine (conventional therapy) to maintain HbA1c levels at <6.5%. Participants were randomized consecutively and were matched on age, sex, BMI, initial HbA1c level and diabetes duration. There was no limit on the upper value of HbA1c (the range of initial HbA1c was 6.5–14%). Participants with initial HbA1c <9.0% (36% of participants) and HbA1c ≥ 9.0% were evenly randomized to the two arms. Participants randomized to triple therapy were started on metformin 1000 mg/day, pioglitazone 15 mg/day and exenatide 5 µg twice daily before breakfast and supper. At 1 month, metformin was increased to 2000 mg, pioglitazone to 30 mg and exenatide to 10 µg twice daily. If, at 3 months, HbA1c was >6.5%, pioglitazone was increased to 45 mg. Participants receiving conventional therapy were started on metformin 1000 mg/day. If, at 1 month, fasting plasma glucose (FPG) concentration was >6.1 mmol/l (110 mg/dl), metformin was increased to 2000 mg and glipizide started at 5 mg/day. If, at 2 months, FPG was >6.1 mmol/l (110 mg/dl) or HbA1c was >6.5%, glipizide was increased to 10 mg and then to 20 mg. If, at 3 months, FPG was >6.1 mmol/l (110 mg/dl) or HbA1c >6.5%, glargine insulin was started at 10 units before breakfast, and escalated weekly by 1–5 units (based on FPG and HbA1c levels) to 60 units/day to maintain FPG at <6.1 mmol/l (110 mg/dl). After 3 months, participants were seen every 3 months. FPG, body weight and HbA1c were measured at each follow-up visit and medication dose was adjusted to maintain FPG at <6.1 mmol/l (110 mg/dl) and HbA1c at <6.5%, unless hypoglycaemia (blood glucose <3.3 mmol/l (60 mg/dl) or symptoms) was present. Hypoglycaemia was defined as blood glucose concentration <3.3 mmol/l ( 60 mg/dl). If HbA1c increased to >6.5% on two consecutive visits (3 months apart, to ensure that the deterioration in glycaemic control was genuine and not attributable to transient factors) despite maximum antihyperglycaemic therapy, treatment was defined as having failed for that participant. All baseline studies were repeated in participants with treatment failure at the time of declaration of treatment failure and rescue therapy was started. Rescue therapy was short-acting insulin which was started 4–6 units before each meal and the dose was adjusted based on blood glucose measurements to maintain plasma glucose concentration <7.8 mmol/l (140 mg/dl) 2 h after meals. Rescue therapy in the triple therapy arm was glargine insulin which was started at 6–10 units/day and the dose was adjusted to maintain FPG <6.1 mmol/l (110 mg/dl). Figure S3: 7-point home blood glucose measurements in subjects receiving Triple Therapy and Conventional Therapy. FPG = fasting plasma glucose; 2h-AB = 2 hours after breakfast; BL = before lunch; 2h-AL = 2 hours after lunch; BS = before supper; 2hAS = 2 hours after supper; BT = bedtime. Table S2: Adverse events reported by subjects in the Conventional and Triple Therapy groups during the 24-month follow-up period. Conventional Therapy Triple Therapy Any adverse event 87% 90% Hypoglycemia 46% 15% Edema 1.3% 5.3% GI side effects 21% 33% Cancer 1 0 Death 2 0 Fractures 0 0 Table S5. Medication dose at months 6, 12, and 24 Triple Therapy Combination Therapy 6 mo 12 mo 24 mo 2000 2000 2000 Pioglitazone (mg) 38±8 39±9 39±9 17.4±4 18.0±4 Metformin (mg) Exenatide (mg) 17.6±4 6 mo 12 mo 24 mo 1932±60 1981±41 1981±41 Glipizide (mg) 12.2±7 13.0±7 13.6±7 Glargine (U/day) 23±16 37±19 50±26 Results Participants receiving triple therapy experienced a significantly greater reduction in HbA1c level than those receiving conventional therapy (5.95 vs. 6.50%; p < 0.001). Despite lower HbA1c values, participants receiving triple therapy experienced a 7.5-fold lower rate of hypoglycaemia compared with participants receiving conventional therapy. Participants receiving triple therapy experienced a mean weight loss of 1.2 kg versus a mean weight gain of 4.1 kg (p < 0.01) in those receiving conventional therapy. the results of the present study should be viewed as a proofof-concept the cost-effectiveness evaluation of antidiabetic therapy should not be limited to the medication cost in relation to the short term reduction in HbA1c level, but should include other beneficial metabolic effects of each therapy that affect the risk of long-termdiabetic complications; for example, the durability of reduction ofHbA1c, risk of hypoglycaemia, weight loss and reduction of CVD risk factors. Conclusion The results of this exploratory study show that combination therapy with metformin/pioglitazone/exenatide in patients with newly diagnosed T2DM is more effective and results in fewer hypoglycaemic events than sequential add-on therapy with metformin, sulfonylurea and then basal insulin. Message DeFronzo先生が2009年頃からずっと推奨されて きたTriple治療の論文! 今はDPP4阻害薬やSGLT2阻害薬が出てGLP-1受容 体作動薬がない方が一般的かもしれない。 単剤を増やすより、最初から併用は今では当た り前だが。コスト面がどうかはまた議論がある だろう。