PowerPoint - 埼玉医科大学総合医療センター 内分泌・糖尿病内科
advertisement

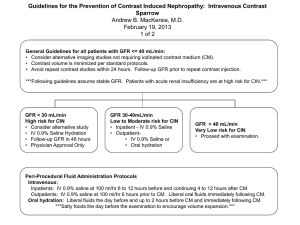
Journal Club The DCCT/EDIC Research Group Intensive Diabetes Therapy and Glomerular Filtration Rate in Type 1 Diabetes. N Engl J Med. 2011 Nov 12. [Epub ahead of print] 2011年11月24日 8:30-8:55 8階 医局 埼玉医科大学 総合医療センター 内分泌・糖尿病内科 Department of Endocrinology and Diabetes, Saitama Medical Center, Saitama Medical University 松田 昌文 Matsuda, Masafumi 1型糖尿病 DCCT/EDIC -42% (P=0.02) 0.12 心 血 管 イ ベ ン ト の 累 積 発 生 率 0.10 従来療法群(N=589) 0.08 0.06 A1C 7.4% vs 9.1% A1C 8.0% vs 8.2% 0.04 0.02 強化療法群(N=593) 0.00 0 1 2 3 4 5 No. at Risk 強化療法群 従来療法群 705 714 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 観察期間(年) 683 688 629 618 113 92 N Engl J Med 2005; 353: 2643-53. Diabetic retinopathy cumulative incidence Secondary prevention 76% conventional 54% Incidence Incidence Primary prevention conventional intensive intensive years years conventional conventional intensive intensive 4 FIG. 2. Estimated cumulative incidence of further 3-step progression of retinopathy from DCCT closeout, by DCCT treatment group, through EDIC year 4, for adolescents (A) and for adults (B); through EDIC year 10, for adolescents (C) and for adults (D). Subjects with prior scatter photocoagulation during DCCT (7 adolescents and 29 adults) were excluded from analyses. Based on Weibull regression models adjusted for the level of retinopathy at the end of the DCCT, primary vs. secondary cohort, the A1C value on entry to the DCCT, and diabetes duration at DCCT baseline. Hazard reduction was for intensive therapy compared with conventional therapy. Diabetes 59:1244–1253, 2010 Ian H. de Boer, M.D., University of Washington, Seattle; Wanjie Sun, M.S., Patricia A. Cleary, M.S., and John M. Lachin, Sc.D., The George Washington University, Rockville, MD; Mark E. Molitch, M.D., Northwestern University, Chicago; Michael W. Steffes, M.D., Ph.D., University of Minnesota, Minneapolis; and Bernard Zinman, M.D., Samuel Lunenfeld Research Institute, Mount Sinai Hospital, University of Toronto, Toronto 10.1056/nejmoa1111732 nejm.org Background An impaired glomerular filtration rate (GFR) leads to end-stage renal disease and increases the risks of cardiovascular disease and death. Persons with type 1 diabetes are at high risk for kidney disease, but there are no interventions that have been proved to prevent impairment of the GFR in this population. Methods In the Diabetes Control and Complications Trial (DCCT), 1441 persons with type 1 diabetes were randomly assigned to 6.5 years of intensive diabetes therapy aimed at achieving nearnormal glucose concentrations or to conventional diabetes therapy aimed at preventing hyperglycemic symptoms. Subsequently, 1375 participants were followed in the observational Epidemiology of Diabetes Interventions and Complications (EDIC) study. Serum creatinine levels were measured annually throughout the course of the two studies. The GFR was estimated with the use of the Chronic Kidney Disease Epidemiology Collaboration formula. We analyzed data from the two studies to determine the long-term effects of intensive diabetes therapy on the risk of impairment of the GFR, which was defined as an incident estimated GFR of less than 60 ml per minute per 1.73 m2 of body-surface area at two consecutive study visits. Figure 1. Cumulative Incidence of an Impaired Glomerular Filtration Rate, According to Treatment Group. An impaired glomerular filtration rate (GFR) was defined as a sustained estimated GFR of less than 60 ml per minute per 1.73 m2 of body-surface area. The cumulative incidence of an impaired GFR is shown according to the group to which the participants had been randomly assigned in the Diabetes Control and Complications Trial, with death accounted for as a competing risk. The hazard ratio and P value were calculated with the use of a Cox proportional-hazards model with a robust estimate of confidence limits according to the method of Lin and Wei16 and the robust Wald test. Cell contents are mean (SD) for time between measurements and absolute values of iothalamate GFR or difference (95% confidence interval) for differences in iothalamate GFR within and between groups. Differences with confidence intervals and P values are generated from the General Linear Model. GFR was measured by iothalamate clearance periodically during the DCCT and at years 1 or 2 of EDIC (half of the cohort per year) using standardized methods (Kidney Int 1995;47:1703-20). EDIC baseline is equivalent to DCCT closeout. Results Over a median follow-up period of 22 years in the combined studies, impairment of the GFR developed in 24 participants assigned to intensive therapy and in 46 assigned to conventional therapy (risk reduction with intensive therapy, 50%; 95% confidence interval, 18 to 69; P = 0.006). Among these participants, end-stage renal disease developed in 8 participants in the intensive-therapy group and in 16 in the conventionaltherapy group. As compared with conventional therapy, intensive therapy was associated with a reduction in the mean estimated GFR of 1.7 ml per minute per 1.73 m2 during the DCCT study but during the EDIC study was associated with a slower rate of reduction in the GFR and an increase in the mean estimated GFR of 2.5 ml per minute per 1.73 m2 (P<0.001 for both comparisons). The beneficial effect of intensive therapy on the risk of an impaired GFR was fully attenuated after adjustment for glycated hemoglobin levels or albumin excretion rates. Conclusions The long-term risk of an impaired GFR was significantly lower among persons treated early in the course of type 1 diabetes with intensive diabetes therapy than among those treated with conventional diabetes therapy. (Funded by the National Institute of Diabetes and Digestive and Kidney Diseases and others; DCCT/EDIC ClinicalTrials.gov numbers, NCT00360815 and NCT00360893.) Message/Comments 1型糖尿病患者1375人を対象に、血糖値正 常化を目指す強化療法と、高血糖症状を防 ぐ従来療法を無作為化試験で比較し、その 後の観察疫学研究で評価(DCCT/EDIC試 験)。平均22年の追跡期間の結果、糸球体 濾過率(GFR)低下の長期リスクは早期に 強化療法を受けた群で有意に低く、強化療 法によるリスク低下率は50%だった。