Perioidicty Slide Show 2011
advertisement

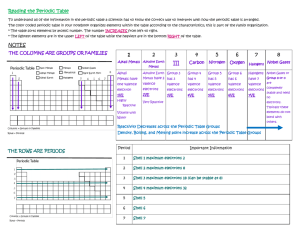
Periodicity Topic 3 Chapter 3 page 75 3.1 The Periodic Table 3.1.1 Describe the arrangement of elements in the periodic table in order of increasing atomic number. 3.1.2 Distinguish between the terms group and period. 3.1.3 Apply the relationship between electron arrangement of element and their position in the periodic table up to Z = 20. 3.1.4 Apply the relationship between the number of electrons in the highest occupied energy level for an element and its position in the periodic table. THE PERIODIC TABLE – THE CHEMIST`S , MOST IMPORTANT TOOL Periodic Table Metals and non-metals are separated by the zigzag line on most periodic tables. Metallic behaviour changes gradually among the elements; the most metallic acting element is Fr on the bottom left of the periodic table. •Periodicity • • Using the simple metal/non-metal classification is possible but it is really too general to be of much use. When the elements are ordered by increasing atomic number a repeating patterns of physical and chemical properties known as periodicity can be observed. The Periodic Table 1871 • This was first discovered by Dmitri Mendeleev (Russian) who is considered the father of the periodic table because, unlike those before him: • • he included 63 known elements and left spaces or gaps which he predicted would be filled in one day by newly discovered elements (three of these gallium, scandium and germanium were found in his lifetime) his table allowed for the accommodation of future findings including noble gases (new column in 1884-1901), the rare earth elements, Bohr atom and electronic structure in 1913 (energy levels), and the discovery of synthetic elements 1939 to present •Groups vs. Periods • • rows are called periods • the period number = number of electron shells columns are called groups or families • the group number = number of valence electrons • groups have similar chemical and physical properties. IB numbering system1-7,0 Activity Arrangement of Groups and Periods •Important Groups –Colour on your periodic table Group 1- Alkali metals Group 2- Alkaline Earth Metals Group 3 – Boron Family Group 4 – Carbon Family Group 5 – Nitrogen Family Group 6 – Oxygen Family Group 7 – Halogens Group 0 – Nobel Gases Assigned Work • • Use the class notes Periodicity to complete Introduction to Matter and Periodicity Practice Questions Response to Video 3.2 3.2.1 3.2.2 3.2.3 3.2.4 Physical Properties Define the terms first ionization energy and electronegativity. Describe and explain the trends in atomic radii, ionic radii, first ionization energies, electronegativities, and melting points for the alkali metals (Li → Cs) and the halogens (F→I). Describe and explain the trends in atomic radii, ionic radii, first ionization energies, and electronegativities for elements across period 3. Compare the relative electronegativity value of two or more elements based on their position in the periodic table. Five Physical Trends For each of the following be able to i) define the term ii) state the trend (if there is an decrease or increase) as you go across a period and down a group, and iii) explain why the trend occurs. 1. atomic radii 2. ionic radii 3. first ionization energies 4. electronegativities 5. melting points 1. Atomic Radii • • Atoms do not have definite boundaries; the boundary is where you are likely to find the outer electrons that are moving around the nucleus. Since this is a moving target... We measure the covalent atomic radius (the distance of half way between the nuclei of two atoms in a diatomic molecule). Atomic Radii Trends • Within each period, the atomic radius tends to decrease with increasing atomic number. • Within each group, the atomic radius tends to increase with the period number in part because there are more layers (energy levels) and in part because shielding (next slide) increases. • Thus, the largest atom is cesium and the smallest fluorine. Nuclear Charge and Shielding • Shielding electrons are the electrons in the energy levels between the nucleus and the valence electrons. They are called "shielding" electrons because they "shield" the valence electrons from the force of attraction exerted by the positive charge in the nucleus. shielding electrons valence electron Nuclear Charge and Shielding • As you go down a group the shielding effect increases since there are more energy levels (shields). This results in the outer electrons being held less tightly. • The strength of the attraction between the nucleus and the outermost electrons helps determines the atomic radius. The stronger the attraction, the smaller the size of the atom. The weaker the attraction (such as from shielding), the larger the atom or ion. Shielding Analogy- A concert Nuclear Charge and Shielding • Effective nuclear charge (ENC) is the charge felt by the valence electrons after you have taken into account the number of shielding electrons that surround the nucleus. Look at your periodic table. ELEMENT nuclear charge shielding (number of protons) electrons valence electrons ENC protons-shielding e- Li 3 2 1 3-2 = +1 O 8 2 6 8-2 = +6 F 9 2 7 9-2 = +7 Ne 10 2 8 10-2 = +8 • As you go across a period, the number of protons and effective nuclear charge (i.e. pull on valence e-) increases while shielding does not change. This results in outer electrons being pulled in closer to the nucleus and smaller atoms being found on the right side of the periodic table. • Nuclear charge and shielding must be considered together. Nuclear Charge and Shielding • • As you go down a group, the increase in the nuclear charge is cancelled out by the increase in shielding electrons and the effective nuclear charge (ENC) stays the same. The extra layers of electrons result in the outer electrons being further away from the nucleus and the lack of a stronger ENC to pull them closer to the nucleus results bigger atoms being found at the bottom of a group ELEMENT • nuclear charge (number of protons) shielding electrons valence electrons Effective Nuclear Charge protons-shielding e- F 9 2 7 9-2 = +7 Cl 17 10 7 17-10=+7 Br 35 28 7 35-28 =+7 As ENC ↓ size ↑; as ENC ↑ Size ↓ 2. Ionic Radii Trends • • Cations are always smaller than their neutral atoms. Loss of outer electrons results in an increase in nuclear charge on the remaining ones. Anions are always larger than their neutral atoms. Effective nuclear attraction does not change for an increased number of electrons. 11-10= +1 11-2 = +9 (now there are 8 valence) 17- 10 =+7 17-10 = +7 (but there is one more electron in valence) Ionic Radii Trends cont’d • • • Across a period the cations decrease in radius. With each increase in charge, (i.e. Na1+, Mg2+, etc.), the atoms contract because of the greater attractive force of the nucleus. When you reach the nonmetals (anions) there is an abrupt increase in radii and then the radius again decreases. 3. Ionization Energy • • • • The minimum energy needed to remove the outermost electron from a neutral atom in the gaseous state is called the first ionization energy. Equation for first ionization energy: Ca(g) + energy (E1) —> Ca+ (g) + e‾ As you go across a period the ionization energies increase (effective nuclear charge is increasing but the shielding effect is constant, so more energy is needed to pull the electrons from the atom). Nobel gases do not form ions. They have a full outer valence of 8 e- and are stable. 4. Electronegativity • • • The electronegativity of an element is the tendency for an atom to attract a bonding pair of electrons to itself when it is chemically combined (covalently bound) with another element. It is a measure of how strongly an atom can attract an electron to itself. Electronegativities are expressed in arbitrary units on the Pauling Electronegativity scale (1-4). We will see more of electronegativity in the next unit. Electronegativity Trends • Electronegativity increases going across the period as the attraction of the nucleus to the electrons increases, and decreases down each group. • F is the most electronegative element and Cs is the least. Summary Summary •atomic radius decreases • ionic radius decreases • ionization energy increases • electronegativity increases • metallic character decreases INCREASING EFFECTIVE NUCLEAR CHARGE 5. Melting Points Period 3 Trends • • • Physical properties, such as melting point, boiling point and density depend on the nature of the bonding between the particles of the element which we will look at in the next section. The more metallic part of the period will have a higher melting/boiling point due to stronger bonds. The nonmetallic tend to have a lower melting/boiling point but the pattern is more complex. Melting Points/Boiling Points Trends Left • At the left of the period the elements (Li, Na, K) are solid metals whose atoms are held together tightly by strong bonds (metallic bonds). As you go across the across the period table, the strength of the metallic bonding increases which results in increasing melting points from left to right Melting Points/Boiling Points Centre • At the centre of period 2 and 3, we find C and Si, giant covalently bonded structures which have the strongest bonds of all substances (Cn is a diamond), and therefore the highest melting points. Melting Points/Boiling Points Right • Following C and Si, the melting points suddenly drop as the elements (N, P) have forces holding one molecule (eg. Cl2) to another molecule (another Cl2). • Melting points are low, and depend on mass and size of the molecules P4, S8, or Cl2. This is further emphasised with the noble gases (He, Ne, Ar), which exist as single atoms with very, very weak forces between the gas molecules and very low melting points. Assigned Work • • Work on Physical Periodic Trend Practice Questions Spreadsheet Lab-40 min | PERIODIC TRENDS OF ALKALI METALS, HALOGENS, AND PERIOD 3 ELEMENTS 3.3 Chemical Properties 3.3.1 Discuss the similarities and differences in the chemical properties of elements in the same group. Know the following reactions: • Alkali metals (Li, Na, and K) with water • Alkali metals (Li, Na, and K) with halogens (Cl2, Br2 and I2) • Halogens (Cl2, Br2 and I2) with halide ions (Cl¯, Br¯, and I¯) Review – Balancing Chemical Equation • • • • • See handbook on line under General Info This slide show is on blog Worksheet Balancing Chemical Reactions Handout Classifying Chemical Reactions (focus on single and double displacement and writing ionic equations) Chemical Reactions Lab Alkali Metals • • • • • Most metallic character Highly reactive (only have 1 outer electron to lose)-therefore not found in nature in elemental state; reacts with O2, to form oxide coating and H2O to form basic (alkali) solutions. Increase in reactivity down the group (Fr is most reactive metal). Softest of all metals (can be cut with a knife) and decrease in melting point down the group. Decrease in density down the group (atoms are larger and less can be packed in a space). Alkali Metals Chemical Trends 1. React exothermically (give off heat) with H2O to form a metal hydroxide solution and hydrogen gas: • e.g. 2Na (s) + 2HOH(l) →2NaOH(aq) + H2(g) • These elements are called alkali metals because they produce OH¯ when combined with H2O which makes resulting solution alkaline (basic) NaOHaq) → Na+(aq) + OH-(aq) 2. alkali metals are donate electrons while halogens accept electrons. Therefore these metals combine directly with halogens (Cl2, Br2 and I2), to form ionic salts). 3. • 2Na(s) + Cl2(g) → 2NaCl(s) • 2K(s) + Br2(l) →2KBr(s) react to O2 in air to form a solid, white or greyish surface coating of metal oxide 4Li(s) + O2(g) →2Li2O(s) Rust, corrosion and oxides • • Al is an extremely important metal since it is light and strong (can be used to make airplanes) and cheap enough to be found in every kitchen. Its big advantage over steel (also very strong but contains iron) is that is does not rust. It is extremely reactive with air (even more so than iron) but unlike iron (III) oxide (rust), aluminum oxide is a tough protective coating that is harder than the Al itself and prevents the metal from further corrosion. Al(s) + 3O2(g) →2Al2O3(s) Rust, corrosion and oxides • Fe2O3 is a red flaky powder that soon falls off and exposes the metal so fresh corrosion until the Fe is `rusted away`. 4Fe(s) + 3O2(g) → 2Fe2O3(s) •Halogen Properties • • • • • Typical non-metal character Most reactive of non-metals (only need to gain 1 electron to fill outer shell) - not found free in nature (always found combined with other elements or is diatomic). Decrease in reactivity down the group (F2 is most reactive non-metal). Increase in melting point down the group (go from gas → liquid → solid) because forces holding diatomic molecules together are stronger. Distinctive colours, toxic and corrosive Halogen Colours View Periodic Videos • F2 yellowish gas • Cl2 greenish • Br2 • I2 gas reddish brown liquid purplish black solid (SATP) -gas is violet Reactions of Halogens 1. Form ionic compounds by combining with metals e.g. NaF Halogen means salt forming. 2. Form covalent bonds and molecular compounds by binding with other non-metals e.g. F2O 3. Halogens with halogen salts -a halogen higher in group 7 will displace a halogen ion that is lower down the group from solution. (i.e. halogens lower down are less reactive than those above them). Cl2(g) + 2NaBr(aq) →Br2(l) + 2NaCl (aq) Br2(l) + 2NaCl(aq)→ no reaction HALOGEN Br2 HALOGEN ION Cl- Reactions of Halogens 4. Reactions between halide ions and silver solutions; formation of silver halides (AgX) Ag+ (aq) + X ¯(aq) → AgX (s) where X = Cl, Br, or I AgNO3 (aq) + NaCl(aq) → AgCl (s) + Na(NO3)(aq) • spectator ions Colours of silver halides: Silver halides are used in black and white film photography. • AgCl white AgBr cream AgI yellow Summary of some halogen reactions •Overview of Alkali Metals and Halogens Alkali Metals • • • • • Most metallic character Highly reactive (only have 1 outer electron to lose)-therefore not found in nature in elemental state; reacts with O2, to form oxide coating (tarnish) and H2O to form basic (alkali) solutions. Increase in reactivity down the group (Fr is most reactive metal). Softest of all metals (can cut with knife) and decrease in melting point down the group because atoms are larger (Cs is largest atom) Decrease in density down the group (atoms are larger and less can be packed in a space) Halogens • • • • • Typical non-metal character Most reactive of non-metals (only have 1 electron to gain to have 8 in valence) - not found free in nature (combined with other elements or diatomic). Halogen means salt former; form salt when they bond to alkali metals. Decrease in reactivity down the group (F2 is most reactive non-metal). Increase in melting point down the group because of forces holding diatomic molecules together are stronger. Distinctive colours, toxic and corrosive: • • • • F2: yellowish gas Cl2: greenish gas Br2: reddish liquid I2: purplish, black solid (sublimes to purple gas) •Alkaline Earth Metals and Nobel Gases Group 2- Alkaline Earth Metals • Similar to the Alkali metals but less reactive (but still too reactive to be found in elemental state in nature). Group 0 -Nobel Gases • • • All colourless, odourless gases at SATP Least reactive of all elements; only the heavier ones (Kr, Xe Rn) react with F2 under controlled conditions All except radon (which is radioactive) are nontoxic. •Assigned Work • Worksheet Chemical Properties of Alkali Metals and Halogens • Chemical Periodicity Practice MC • Chemical Reactions Lab • Activity Series Lab