Lab Activity 10 Purification of LDH from Chicken Part I
advertisement

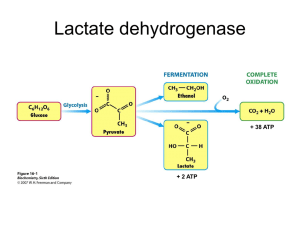
Lab Activity 10 Purification of LDH from Chicken Part I IUG, Fall 2012 Dr. Tarek Zaida 1 Background • LDH catalyzes the nicotinamide cofactordependent interconversion of lactate and pyruvate: 2 • LDH is found in almost all organisms because it plays an important role in carbohydrate metabolism during conditions in which pyruvate production from glycolysis exceeds the ability of the cell to metabolize the pyruvate, • LDH converts the pyruvate to lactate, and thereby regenerates the oxidized NAD required for further glycolysis. • LDH also allows the conversion of lactate to pyruvate; • both the pyruvate and NADH produced can then be used for other processes. 3 LDH activity is readily measurable: • The extinction coefficient at 340 nm of NADH is much higher than that of NAD. • If the only substrates added to the reaction are NAD and lactate (or NADH and pyruvate), the change in absorbance at 340 nm should be proportional to the change in NADH concentration due to the LDH activity present in the cuvette. 4 5 • In any protein purification protocol it is necessary to take advantage of the way in which the protein of interest (in this case, LDH) differs from the other proteins in the mixture. • Most tissues contain thousands of proteins; you need to use the properties of LDH to separate it from all of the other proteins present. 6 • Most tissues contain proteases (enzymes that degrade other proteins). • Avoiding proteolytic damage to your protein can be difficult. 7 • Three techniques are commonly used to keep proteolysis to a minimum: 1) perform the purification in the presence of protease inhibitors, 2) perform the purification at low temperatures (4°C or on ice), and 3) perform the purification in the minimal amount of time possible. Because it is not easy to do the last of these (the purification procedure will take more than one lab period), you should keep your sample on ice or in the refrigerator as much as possible. 8 Definitions • Tris-(hydroxymethyl) aminomethane hydrochloride (Tris-HCl) is a commonly used buffer, and is intended to help control the pH of the solution. • Tris is inexpensive and generally inert in biochemical experiments. • One drawback is the fact that the pKa of Tris changes by –0.031 pH units per °C, and therefore the pH of a Tris-buffered solution is very temperature-dependent. 9 • 2-Mercaptoethanol (b-ME) is a reducing agent; it prevents formation of disulfide bonds between free cysteine residues. • It also inhibits some proteases. • Phenylmethylsulfonyl fluoride (PMSF) is an irreversible inhibitor of serine proteases. • PMSF is toxic; avoid getting PMSF on your skin. • Ethylenediamine tetraacetic acid (EDTA) is a chelating agent; it is used to remove metal ions from solution. • Some proteases are dependent on metal ions (especially calcium ions), so EDTA acts as an inhibitor of some proteases. 10 Experiment Reagents 11 Procedure 1. Tissue preparation– Cut ~50 g of muscle tissue from the tissue source (record the exact weight of tissue used). Cut the tissue into small pieces with scalpel or razor blades. Discard the connective tissue and fat. 2. Soluble protein extraction – Place the minced tissue and 75 ml of cold Extraction Buffer in a blender, and put the top on the blender. 12 Disrupt the tissue by homogenizing. Use 4 x 30 second bursts, with at least 10 seconds in between each burst to allow the temperature of the homogenate to decrease. 3. Centrifugation– Put the homogenized tissue/buffer mixture into four pre-chilled 50 ml centrifuge tubes (note: the mixture will be the consistency of a thick milk shake, so a spatula will help). Balance the tubes (i.e . make sure that each pair of tubes have the same mass). Make sure that the tubes are not too full (you do not want to spill your mixture inside the rotor). Centrifuge your homogenate for 20 minutes at 15,000 rpm. 13 14 4. Filtration– Pour the supernatant (i.e . the fluid on top) through two layers of cheesecloth into a pre-chilled beaker. The cheesecloth removes lipids from the solution; the filtration step is much easier if you put the cheesecloth into a funnel). • Discard the cell debris pellets. Measure and record the volume of the supernatant. 15