Gases in Seawater
advertisement

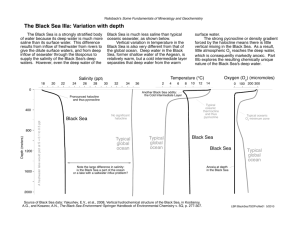
Seawater Salinity, Density, Temperature Water molecule unique in structure and properties H2O is the chemical formula for water. Unique properties of water include: Higher melting and boiling point than other hydrogen compounds. High heat capacity, amount of heat needed to raise the temperature of one gram of water 1oC Latent (hidden) heat = energy that is either absorbed or released as water changes state Can dissolve more substances than any other solvent Called universal solvent Water molecules are asymmetrical in shape with the two hydrogen molecules at one end H+ separated by 105o when gas or liquid phase and 109.5o when ice Interconnections of water molecules Polarity causes water molecules to form weak (hydrogen) bonds between water molecules Water sticks to itself and to other substances Allows water to be the universal solvent Figure 5-3 Hydrogen bonds in H2O Figure 5-8 Pure water vs Seawater pH = 7.0 for pure water pH= 8.1 for seawater, slightly alkaline Density = 1.000 g/cm3 for pure water Density = 1.028 for seawater Freezing point = 0 °C or 32° F for pure water -1.9 °C or 28.6 °F for seawater Boiling point= 100°C or 212°F for pure water 100.6 °C or 213°F for seawater Snowflake geometry All snowflakes have 6-sided geometry Caused by water’s polarity and ability to form hydrogen bonds Why does ice float on water? The formation of ice As water cools to 4°C: Molecules slow Water contracts Density increases Below 4°C: Hydrogen bonds form Water expands As water freezes: Expands by 9% Water as a solvent Water dissolves table salt (NaCl) by attracting oppositely charged particles Pulls particles out of NaCl structure to dissolve it Figure 5-4 Salinity Salinity = total amount of solid material dissolved in water Can be determined by measuring water conductivity Typically expressed in parts per thousand ppt or (‰) Major dissolved components in seawater: Constituents of ocean salinity 35 g of salt in 1000 g of seawater Addition of salt modifies properties of water Pure water freezes at 0oC. Adding salt increasingly lowers the freezing point because salt ions interfere with the formation of the hexagonal structure of ice. Vapor pressure decreases as salinity increases because salt ions reduce the evaporation of water molecules. Density of water increases as salinity increases. Change in scale Graph of Density of freshwater: Seawater density depends on temperature, salinity and pressure! Therefore, it increases with > salt content at constant temp high density in cold, salty waters –why is this important? Surface salinity variation Pattern of surface salinity: Lowest in high latitudes Highest in the tropics Dips at the Equator Surface processes help explain pattern Surface salinity variation High latitudes have low surface salinity High precipitation and runoff Low evaporation Tropics have high surface salinity High evaporation Low precipitation Equator has a dip in surface salinity High precipitation partially offsets high evaporation Why is surface Atlantic more salty than Pacific? Salinity map showing areas of high salinity (36 o/oo) in green, medium salinity in blue (35 o/oo), and low salinity (34 o/oo) in purple. Salinity is rather stable but areas in the North Atlantic, South Atlantic, South Pacific, Indian Ocean, Arabian Sea, Red Sea, and Mediterranean Sea tend to be a little high (green). Areas near Antarctica, the Arctic Ocean, Southeast Asia, and the West Coast of North and Central America tend to be a little low (purple). http://www.biosbcc.net/ocean/marinesci/02ocean/swcomposition.htm Salinity variations Location/type Salinity Normal open ocean Baltic Sea Red Sea Great Salt Lake Dead Sea Tap water Premium bottled water 33-38‰ 10‰ (brackish) 42‰ (hypersaline) 280‰ 330‰ 0.8‰ or less 0.3‰ Decrease Salinity by: Precipitation – rain, sleet, snow, falls directly on ocean Runoff-rivers carry fresh water to the ocean Icebergs melting – when glacial ice breaks off –this is mostly fresh water Sea ice melting with the spring thaw-this has a little salt but is mostly water Increase salinity by: Sea ice forming in cold ocean areas as water freezes– 30% of salts in seawater are retained in ice Evaporation – removes very pure water, all salts are left behind, occurs in hot climates Sea water consists of water with various materials dissolved within it. The solvent is the material doing the dissolving and in sea water it is the water. The solute is the material being dissolved. Solutes in seawater = organic compounds and nutrients, ionic salts, dissolved gases and trace elements Salinity = salts dissolved in seawater Solutes in water: Nutrients and Organics Major nutrients in the sea are compounds of nitrogen, phosphorus and silicon. Nutrients are chemicals essential for life Because of usage, nutrients are scarce at the surface and their concentrations are measured in parts per million (ppm). Concentration of nutrients vary greatly over time and because of this are considered a nonconservative property Marine organic compounds occur in low concentrations consist of large complex molecules, such as fat, proteins, carbohydrates, hormones and vitamins produced by organisms or through decay Solutes in water: Ionic salts Sodium and chlorine alone comprise about 86% of the salt in the sea. major constituents of salinity display little variation over time 99% of all the salt ions in the sea are sodium (Na+), chlorine (Cl-), sulfate (SO4-2), Magnesium (Mg+2), calcium (Ca+2) and potassium (K+) Solutes in water: Gases and Trace elements In order of decreasing abundance the major gases in the sea are nitrogen, oxygen, carbon dioxide and the noble gases, argon (Ar), neon (Ne) and helium (He). Nitrogen and the noble gases are considered to be inert because they are chemically non-reactive. Trace elements occur in minute quantities and are usually measured in parts per million (ppm) or parts per billion (ppb). • Even in small quantities they are important in promoting life or killing it. Salinity in the ocean: steady-state condition because amount of salt added to the ocean (input from source) equals the amount removed (output into sinks) Salt sources include weathering of rocks on land and the reaction of lava with sea water. Salt sinks include the following: Evaporation removes only water molecules. Remaining water becomes increasingly saline, producing a salty brine If enough water evaporates, the brine becomes supersaturated and salt deposits precipitate forming evaporite minerals Wind-blown spray carries minute droplets of inland Adsorption of ions onto clays Shell formation by organisms Cycling of dissolved components in seawater Oceans’ salinity may have increased over time Gases in Seawater The solubility and saturation value for gases in sea water increase as temperature and salinity decrease and as pressure increases. The surface layer is usually saturated in atmospheric gases because of direct exchange with the atmosphere. Below the surface layer, gas content reflects relative importance of respiration, photosynthesis, decay and gases released from volcanic vents. Gases in Seawater: O2 Surface layer is rich in oxygen because of photosynthesis and contact with the atmosphere. Oxygen minimum layer occurs at about 150 to 1500m below the surface and coincides with the pycnocline. Sinking food particles settle into this layer and become suspended in place because of the greater density of the water below food draws large numbers of organisms which respire, consuming oxygen. Decay of uneaten material consumes additional oxygen Gases in Seawater: O2 Density difference prevents mixing downward of oxygen-rich water from the surface or upwards from the deep layer Deep layer is rich in oxygen because its water is derived from the cold surface waters which sank to the bottom Consumption is low because there are fewer organisms and less decay consuming oxygen Anoxic waters contain no oxygen and are inhabited by anaerobic organisms (bacteria) Oxygen tends to be abundant in the surface layer and deep layer bottom, but lowest in the pycnocline. Gases in Seawater: CO2 Major sources of carbon dioxide are respiration and decay Major sinks are photosynthesis and construction of carbonate shells Carbon dioxide controls the acidity of sea water Dissolved CO2 in water acts as a buffer, a substance that prevents large shifts in pH Gases in Seawater: CO2 Dissolution of carbonate shells in deep water results because cold water under great pressure has a high saturation value for CO2 and the additional CO2 releases more H+ ions making the water acid. Warm, shallow water is under low pressure, contains less dissolved CO2 and is less acidic. Carbonate sediments are stable and do not dissolve. Gases in Seawater: CO2 pH is related to the amount of CO2 dissolved in water because it combines with the water to produce carbonic acid which releases H+ ions. CO2 + H2O H2CO3 H+ + HCO3- H+ + CO3-2 H2CO3 is carbonic acid, HCO3- is the bicarbonate ion and CO3-2 is the carbonate ion. Adding CO2 shifts the reaction to the right and produces more H+ ions making the water more acid. Removing CO2 shifts the reaction to the left, combining H+ ions with carbonate and bicarbonate ions reducing the acidity. Ocean buffering Ocean pH = 8.1 (slightly basic) Buffering protects the ocean from experiencing large pH changes Water samples must be collected in inert containers and isolated during recovery to prevent contamination Nisken bottle has valves at each end which are automatically closed when a weight is sent down the cable causing the bottle to flip over and seal itself Sample depth can be determined from cable inclination and length or with a pulsating sound source Collection of water samples Temperature, Salinity, and Water Density The relationship between temperature, salinity and density of seawater. Temperature vs Heat Temperature is the measure of how fast the molecules in a substance are moving Temperature measured in Kelvin, Celsius, Fahrenheit Heat is a measure of how much energy has to be put into a system or removed from a system to change its temperature or state (solid, liquid, or gas) Heat has units of Energy (1 calorie, calor = heat; the amount of heat required to raise the temp. of 1 gram of water by 1 C°) Ocean moderates coastal temperatures Water has high heat capacity it can absorb or release large quantities of heat without changing temperature Hydrogen bonds cause thermal inertia Moderates coastal temperatures Evaporation occurs at temp < 100° C But it requires more energy to do so Atmospheric transport of surplus heat from low latitudes into heat deficient high latitudes areas: Seawater density Factors affecting seawater density: Temperature ↑, Density ↓ (inverse relationship) Salinity ↑, Density ↑ Pressure ↑, Density ↑ Temperature has the greatest influence on surface seawater density Density and temperature variations with depth Pycnocline and thermocline Pycnocline = layer of rapidly changing density Thermocline = layer of rapidly changing temperature Present only in low latitude regions Barrier to vertical mixing of water and migration of marine life Pyncnocline graphics http://www.youtube.com/watch?v=TxdiU3LJlZ8 From Scripps about 11 min Thermocline graphics Swimming through a thermocline 40 sec http://www.youtube.com/watch?v=PP42k7YD-nw http://www.youtube.com/watch?v=204cv98oduI Thermocline explained related to hurricanes http://www.youtube.com/watch?v=aKN7Tq_-uwQ 15 minutes on oceans and motions Thermocline http://www.youtube.com/watch?v=ovIvtKSQy9Y Thermocline circulation at risk Ocean layering based on density Mixed surface layer (surface to 300 meters) Low density; well mixed by waves, currents, tides Upper water (300 to 1000 meters) Intermediate density water containing thermocline, pycnocline, and halocline (if present) Deep water (below 1000 meters) Cold, high density water involved in deep current movement Salinity variation with depth Curves for high and low latitudes begin at different surface salinities Halocline = layer of rapidly changing salinity At depth, salinity is uniform Figure 5-22 Halocline graphics Diving through a halocline 45 sec http://www.youtube.com/watch?v=EUxjx_f-r9A http://www.youtube.com/watch?v=dHn80f3lAUs BbC halocline 1 min scuba diving in florida caves http://www.youtube.com/watch?v=oBCtuaWKjlU Arctic sea ice has grown to a record breaking amount