Photosynthesis
advertisement

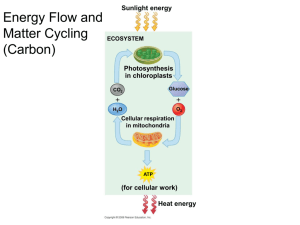
Photosynthesis CHAPTER 10 Sunlight as an Ultimate Energy Source All living things need energy Photosynthesis provides this energy Converts light energy into chemical energy Acquired by either autotrophic or heterotrophic means Autotrophs Live without consuming anything from other living things Require water, soil minerals, and CO2 Producers of the Heterotrophs Live on compounds produced by other organisms Consumers of the biosphere biosphere Photoautotrophs Use light as energy sources E.g. plants, algae, protists, and bacteria Eat living organisms for energy E.g. animals Decomposers of the biosphere Breaks down dead organic matter E.g. fungi Anatomy of a Leaf Stomata allow gas exchange Veins move water from roots to leaves and sugars from leaves to roots Chloroplasts, the site of photosynthesis, Located in the mesophyll or interior leaf tissue All green areas of plants, concentrated in leaves Chloroplasts Double membrane bound organelle Fluid filled space called the stroma Contains multiple thylakoids, or interconnected membranous sacs Stacked into grana Chlorophyll pigment within Gives plant characteristic colors Captures energy for photosynthesis Equation of Photosynthesis 6CO2 + 6H2O + sunlight C6H1206 + 6O2 What color line is showing reduction? oxidation? Redox Revisited Cellular Respiration Energy from sugar as electrons from H to O2 = H2O Lose PE as fall to more electronegative oxygen Mitochondria use energy released to make ATP Photosynthesis H20 split and electrons to CO2 = sugar (reduction) Gain PE as bond complexity increases Requires energy = endergonic Light provides boost Photosynthesis: An Overview Light reactions [photo part] Solar energy to chemical energy Light drives transfer of e -’s and H+ NADP+ NADPH (reduction or oxidation?) Create ATP using chemiosmosis to power photophosphorylation NO sugar produced Calvin cycle (dark reaction) [synthesis part] CO2 incorporated into organic molecules, carbon fixation Add e -’s from NADPH and ATP to reduce into carbohydrates Makes sugar Doesn’t need light directly Photosynthesis Understanding Sunlight Electromagnetic energy Exists as discrete packets of particles called photons All wavelengths make up an electromagnetic spectrum Wavelengths are distance between crests of waves and inversely related to amount of energy Visible light most important to life Detectable by human eye Violet end is shortest waves Red end is longest waves All combined = white light Photosynthetic Pigments Light can be reflected, transmitted, or absorbed Chloroplasts vary in pigments Chlorophyll a, b, and carotenoids Violet-blue and red light most efficient for photosynthesis Carotenoids have role in photoprotection In human eye too Action spectrum Excitation of Chlorophyll Absorption of light elevates electrons of pigments to higher orbital ( PE) Pigments absorb in specific range Unstable in upper orbital so ‘fall’ back quickly Releases energy as heat White vs black cars or clothing in the South Photosystems Protein complex with a reaction center surrounded by light-harvesting complexes Chlorophyll a always bound with reaction center molecules Other pigments with lightharvesting complexes Gather light from larger surfaces Pigments absorb photons and transfer to reaction center complex Electrons transferred to primary electron acceptor, reducing it Two types, II and I Light Reaction Occurs in the thylakoids Two Photosystems PS I absorbs at 700nm PS II at 680nm Two electron flow patterns Linear electron flow Cyclic Linear Electron Flow To Calvin cycle Comparing Chemiosmosis Similarities ETC in membranes pump protons across as e-’s moved to more EN carriers ATP synthase utilizes [H+ gradient] Differences M: e-’s from organics, protons move out P: e-’s from H2O, protons move in Calvin Cycle Anabolic reaction in the stroma Products from light reaction are reactants for dark (3) CO2 molecules combine to create (1) 3 carbon sugars (glyceraldehyde 3phosphate, G3P) Cycle must occur 3 times for 1 molecule to be made Broken into 3 steps Carbon fixation Reduction Regeneration of CO2 acceptor (RuBP) CO2 3PG RuBP G3P G3P G3P Carbon Fixation 1 CO2 into stroma Attaches to ribulose bisphosphate (RuBP), a 5 carbon sugar Catalized by rubisco Most abundant protein on Earth Forms unstable 6 carbon molecule Immediately to (2) 3-phosphoglycerate (3PG) 2 for every 1 CO2 molecule Reduction 3PG gains a phosphate from ATP to create 1,3- bisphosphoglycerate NADPH reduces 1,3-bisphosphoglycerate to G3P 3 cycles (3 CO2’s) create 6 G3P Only 1 leaves (3 carbons out) Other 5 recycled (15 carbons remain) Regeneration of CO2 Acceptor 5 G3P are rearranged into 3 RuBP (5 carbons each) Cost 3 ATP Capable of accepting CO2 again Overall cost of cycle 9 ATP 6 NADPH 3 CO2 2 G3P to make sugars and other fuels Review of Photosynthesis