Gr 10 Chemistry - Lesson Plan
advertisement
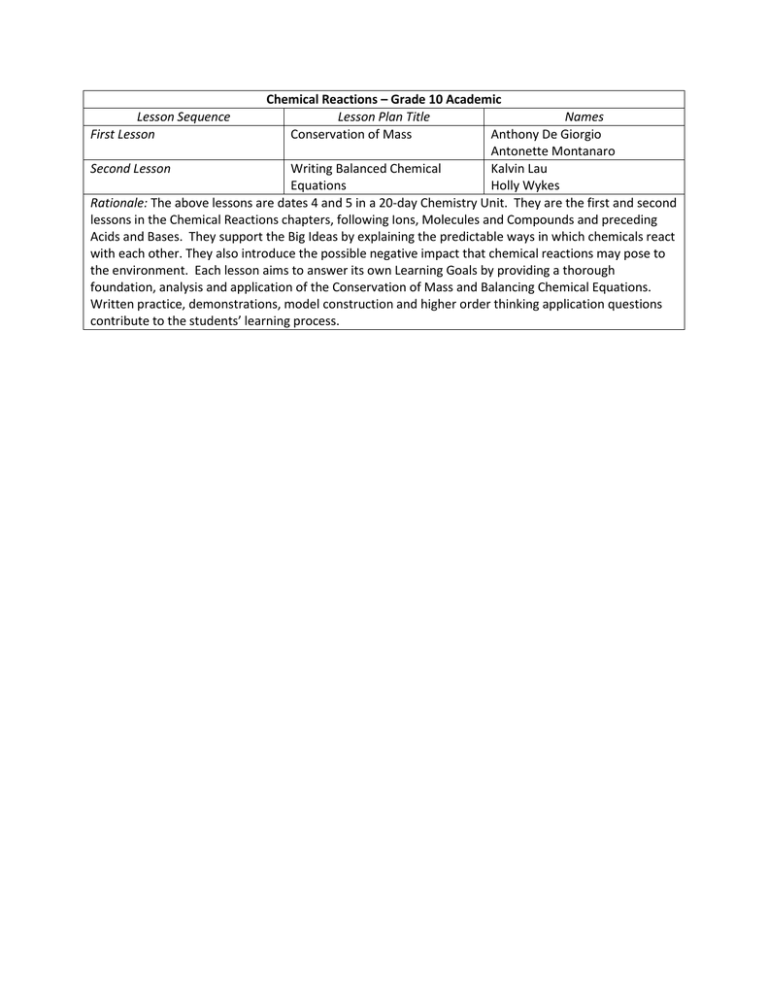
Chemical Reactions – Grade 10 Academic Lesson Sequence Lesson Plan Title Names First Lesson Conservation of Mass Anthony De Giorgio Antonette Montanaro Second Lesson Writing Balanced Chemical Kalvin Lau Equations Holly Wykes Rationale: The above lessons are dates 4 and 5 in a 20-day Chemistry Unit. They are the first and second lessons in the Chemical Reactions chapters, following Ions, Molecules and Compounds and preceding Acids and Bases. They support the Big Ideas by explaining the predictable ways in which chemicals react with each other. They also introduce the possible negative impact that chemical reactions may pose to the environment. Each lesson aims to answer its own Learning Goals by providing a thorough foundation, analysis and application of the Conservation of Mass and Balancing Chemical Equations. Written practice, demonstrations, model construction and higher order thinking application questions contribute to the students’ learning process. Unit Plan Grade 10 Academic: Chemistry 1. Matter and the Periodic Table 2. Ions, Molecules and Compounds 3. Ions, Molecules and Compounds (Cont’d) Quick Lab 4. Chemical Reactions: - Chemical Reactions - Conservation of Mass 5. Chemical Reactions: - Writing Balanced Chemical Equations 6. Chemical Reactions: - Strategies for Balancing Chemical Equations 11. Using Neutralization reactions to Solve Environmental Challenges - Group Activity 7. Quiz: Balancing Chemical Reactions Introduction to Acids and Bases 8. Properties of Acids and Bases - Identifying Acids - Identifying Bases 9. Lab and Report: Acids and Bases 10. Neutralization Reactions - Neutralization 12. Group Presentations 13. Types of Chemical Reactions: - Synthesis Reactions 14. Types of Chemical Reactions: - Decomposition Reactions 15. Combustion Reactions 16. Single Displacement Reactions 17. Double Displacement Reactions 18. Environmental Application 19. Unit Review 20. Unit Test Quiz: Acids and Bases Lab and Report: Combustion Review Sheet Handed Out Theme 1: Matter and the Periodic Table, Ions, Elements & Compounds – Pink Theme 2: Chemical Reactions – Blue Theme 3: Acids and Bases – Purple Theme 4: Types of Chemical Reactions – Green Theme 5: Review and Unit Test - Yellow 2 “Be the change you want to see in the world!” -Mahatma Gandhi NAMES: Anthony De Giorgio and Antonette Montanaro UNIT: Chemical Reactions TITLE OF LESSON: Types of Reactions and Law of Conservation of Mass BIG IDEAS: MATERIALS: - Chemicals react with each other in - Eggs predictable ways. - Skillet - Chemical reactions may have a negative - Ice cubes impact on the environment, but they can - Strainer also be used to address environmental - 2 beakers challenges. - Pop can (one crushed, one not) - 2 teaspoons MINISTRY EXPECTATIONS: - Salt - C2.1 Use appropriate terminology related - Water to chemical reactions, including, but not - 6 Glow-sticks limited to: compounds, product & reactant. - 6 Icepacks - C3.1 Describe the relationship between - Vinegar chemical formulae, composition and the - Baking soda names of binary compounds (e.g., Carbon - Scale dioxide, CO2, has two oxygen atoms and - Play dough (red, white, blue and yellow) one carbon atom). - Appropriate worksheets (B1.0; B2.1; B1.4) - C3.2 Explain, using the law of conservation of mass and atomic theory, the rationale for balancing chemical equations. - C3.3 Describe the types of evidence that indicate chemical change (e.g., changes in colour, the production of gas, the formation of precipitate, the production of absorption of heat, the production of light). STUDENT LEARNING GOALS: APPENDICES: 1. Introduction to terminology and the A1.0 Chemical vs. Physical Change Egg Demonstration: components of chemical reactions. Teachers Notes 2. What makes a chemical reaction? How to B1.0 Physical or Chemical Change Carousel Activity: distinguish what is and what is not a Worksheet chemical reaction. B1.1 Physical or Chemical Change Carousel Activity: 3. Representing chemical reactions through Answer Key the formation of chemical equations. B2.0 Chemical and Physical Changes, Chemical Equations 4. Introduction to law of conservation of mass and Conservation of Mass Chalkboard Notes and balancing chemical equations. B2.1 Chemical and Physical Changes, Chemical Equations and Conservation of Mass Handout B3.0 Law of Conservation of Mass Worksheet B3.1 Law of Conservation of Mass Worksheet: Answer Key C1.0 Chemical Reactions that Affect our Environment PRIOR KNOWLEDGE: Homework Sheet Definitions and examples of: The Periodic Table, Components of an Atom, the Physical Properties of Matter, the Chemical Properties of Matter and Molecular and Ionic Compounds. 3 T/L STRATEGIES RATIONALE A 1) Chemical Vs. Physical Change Allow students observe a Egg Demonstration: physical and chemical MINDS ON A short demonstration of the physical change, and to get them (10-15 change that occurs when you break thinking about how a minutes) and egg shell and the chemical change physical change may be that occurs when you fry an egg. different from a chemical Appendix A1.0 change. B 1) Physical or Chemical Change Allow students to discover Carousel: what constitutes a ACTION chemical change. Allows (15-20 Have six different stations: Three demonstrating physical changes, students to start thinking minutes) three demonstrating chemical about how/why chemical changes. changes are different from Appendix B1.0; Appendix B1.1 physical changes. 2) Chemical & Physical Changes, Provides students with a Chemical Equations and hard copy of the main (15-20 Conservation of Mass Notes: ideas. Allows students to minutes Notes on characteristics of physical consolidation information. changes and chemical reactions, reactants & products and chemical equations. Use concrete materials (model atoms) to demonstrate chemical equations. Appendix B2.0; B2.1 3) Chemical Equations and Gives students a way to (15-20 Conservation of Mass Group “see” chemical equations minutes) Activity: with solid objects and to In groups of 4, students are given reinforce the Law of simple equations to determine the Conservation of Mass: number of atoms on the reactant and “In a chemical reaction, product side of the equation. They are the mass of the products given scales and play-dough to make always equals the mass of sure that the reactants are equal to the reactants”. the products. Following the group activity, there will be a small debrief led by the teacher to discuss findings. Appendix B3.0; B3.1 C 1) Combustion Notes: Extend thinking to analysis CONSOLID- Together, the class will read the and application. ATION & information on the sheet and briefly CONNEC- discuss the questions. TION (5-10 Appendix C1.0 minutes) ASSESSMENT Predict/ Observe/ Explain In groups Think/ Pair/ Share Questioning during notemaking. Think/ Pair/ Share, questioning while taking up the worksheet. Read hand-out and answer questions for homework. NEXT STEPS Writing Balanced Chemical Equations 4 Appendix A1.0 - Minds On Chemical vs. Physical Change Egg Demonstration – Teachers Notes - - Purpose: demonstrate the difference between a physical and chemical change. Display of egg in front of class. Crack the egg into a clear bowl Questions to ask: “I cracked this egg into this bowl, what kind of change do you think I have produced? Physical or Chemical?” Answer: Physical, because I have not changed the state of the egg? I have not changed its molecular structure. I have simply displaced its parts. Predict: “What do you think will happen when I drop this cracked egg into the heated skillet? Will it produce a chemical or physical change?” Observe: Teacher cracks the egg into the skillet, and it cooks. Explain: It is a Chemical change, because the molecular structure of the egg is changing; and will never be able to return back to its original state. 5 Appendix B1.0 - Action Physical or Chemical Change Activity Worksheet Instructions: * Your teacher will put you into number groups. Station number is the same as your group number. * Follow the instructions for that station and document your observations and conclusions in the space provided. * When your teacher says, you will move in a clockwise direction to your next station. Physical or Chemical Changes? Station #1 Station #2 Materials: 2 soda cans are displayed. One of them is crushed, the other is not. Materials: 6 non-cracked glow-sticks Instructions at station: 2. Look at both the crushed soda can and noncrushed soda can. 3. Write down observations about the two cans. 1. Is this a physical or chemical change? Instructions at station: 2. Observe a non cracked glow-stick. 1. Write down your observations. 2. Now carefully crack the glow-stick and observe what happens. 3. Write down observations. 4. Is this a physical or chemical change? 6 Physical or Chemical Changes? Station #3 Station #4 Materials: Ice cube, small strainer and beaker. The small strainer is placed on top of the beaker and the ice in the strainer. Material: Icepack Instructions at station: 1. Look at the ice at this station and the pool of water it is creating. 2. Write down your observations. 3. Is this a physical or chemical change? Instructions at station: 1. Observe and feel a non-cracked ice-pack. 2. Write down your observations 3. Now, carefully crack the icepack. 4. Observe and feel the icepack once more after it has been cracked. 5. Write down your observations 6. Is this a physical or chemical change? 7 Physical or Chemical Changes? Station #5 Station #6 Materials: water, salt, beaker and teaspoon Materials: Vinegar, baking soda, beaker, teaspoon and sink Instructions at station: 1. Fill your beaker half way with water 2. Write down observations 3. Add a teaspoon of salt into the beaker. 4. Write down observations. 5. Is this a physical or chemical change? Instructions at station: 1. Fill your beaker a quarter of the way full. 2. Write down observations. 3. Place beaker in the sink, then add a teaspoon of baking soda to the vinegar. 4. Write down observations. 5. Is this a physical or chemical change? 8 Appendix B1.1 - Action Physical or Chemical Change Activity –Teacher’s Copy Physical Change Stations Chemical Change Stations Station #1 Station #2 Materials: 2 soda cans are displayed. One of them is crushed and the other is not. Materials: 6 non-cracked glow-sticks Instructions at station: * Look at both the crushed soda can and noncrushed soda can. 3. Write down observations about the two cans. 4. Is this a physical or chemical change? Instructions at station: 2. Observe a non-cracked glow-stick. 3. Write down your observations. 4. Now carefully crack the glow-stick and observe what happens. 5. Write down observations. 6. Is this a physical or chemical change? 9 Physical Change Stations Chemical Change Stations Station #3 Station #4 Materials: Ice cube, small strainer and beaker. The small strainer is placed on top of the beaker and the ice in the strainer. Material: Icepack Instructions at station: 5. Look at the ice at this station and the pool of water it is creating. 6. Write down your observations. 7. Is this a physical or chemical change? Instructions at station: 4. Observe and feel a non-cracked ice pack. 5. Write down your observations 6. Now, carefully crack the icepack. 7. Observe and feel the icepack once more after it has been cracked. 8. Write down your observations 9. Is this a physical or chemical change? 10 Physical Change Stations Chemical Change Stations Station #5 Station #6 Materials: water, salt, beaker and teaspoon Materials: Vinegar, baking soda, beaker, teaspoon and sink Instructions at station: 7. Fill your beaker half way with water 8. Write down observations 9. Add a teaspoon of salt into the beaker. 10. Write down observations. 11. Is this a physical or chemical change? Instructions at station: 6. Fill your beaker a quarter of the way full. 7. Write down observations. 8. Place beaker in the sink, and then add a teaspoon of baking soda to the vinegar. 9. Write down observations. 10. Is this a physical or chemical change? 11 Appendix B2.0 - Action Chalkboard Notes: Physical and Chemical Reactions 1) The Egg experiment/demonstration showed us that matter could both physically and chemically undergo change. - The cracking of the shell is a physical change because no new matter is being formed; only the shape is being changed. - The cooking of the egg is a chemical change because bonds are being broken down and new matter is being formed. Also heat is being absorbed making it an endothermic reaction. The Carousel Activity showed us different types of physical and chemical changes. It also helped distinguish between what constitutes a physical versus chemical change. 2) Taking up the Carousel activity Station Action Observation Physical Change Chemical Change 1) Soda Can Crushing of the soda can Change of shape Yes No 2) Glow Sticks Crack the glow sticks The sticks glow No Yes 3) Ice Cubes Melting of the ice Water is formed from the ice melting Yes No 4) Icepack Breaking the icepack Cold is created when pouch is broken No Yes 5) Water and Salt Adding Salt to Water Will create a cloudy look to the water Yes No 6) Vinegar and Baking soda Adding Baking soda to vinegar Will cause bubbles to form No Yes 12 3) Description of a physical change: “Occurs when an object or substance undergoes a change in physical properties but maintains its chemical properties” - Physical Properties: texture, shape, size, colour, volume, mass, weight, taste, smell, density Description of a chemical change: “Occurs when an object or substance is changed or altered, creating a new substance and an energy change (exothermic: releases energy i.e. explosion or endothermic: system absorbs energy i.e. cold pack)” - Chemical Properties: burning, decomposition, neutralization, cooking, oxidation, ripening, combustion Law of Conservation of Mass and Chemical Equations 4 & 5) The Law of Conservation of Mass “In a chemical reaction, the mass of the products always equals the mass of the reactants” To demonstrate this, teacher will use the small, plastic atoms to show that each atom is conserved throughout the reaction All chemical reactions have reactants and products - Reactants are always found on the left side of the equation, and react together to form the Products, which are always found on the right side of the equation The mass of the atoms on the reactant side must always equal the mass the of atoms on the product side of the equationthis adheres to the Law of Conservation of Mass The number of atoms also stays the same in each equation 13 Appendix B2.1 - Action Chemical and Physical Changes, Chemical Equations and Conservation of Mass Handout 1) What did the activities that we just completed demonstrate to us about physical and chemical reactions? The Egg Experiment: The Carousel Activity: 2) Now, lets take up the Carousel Activity. Together, we will fill in the action and result of the change, and whether it was a physical or chemical change. Station Action Result Physical Change Chemical Change 1) Soda Can 2) Glow Sticks 3) Ice Cubes 4) Icepack 5) Water and Salt 6) Vinegar and Baking soda 14 3) Describe the properties of the following and give an example of each: 1) A physical change: 2) A chemical change: 4) What is the Law of Conservation of Mass? Why is it important with regards to chemical equations? 5) What are the elements on the left side of a chemical equation called? The right side? 15 Appendix B3.0 - Action Law of Conservation of Mass Worksheet Instructions: * Fill out the reactants and products/ equations in the spaces provided. * Look at the reactants and in the space provided write out the number of atoms for each element. Do the same for the products. * Weigh the reactants on the scale and record the mass. Do the same for the products. Equation Reactants (R) Number of atoms in R Mass of R Products (P) Number of atoms in P Mass of P C2 + O2 + O2 CO2 + CO2 H2 + H2 + O2 H2O + H2O 6CO2 + 6H2O C6 H12 O6 + 6O2 CH4 + 2O2 CO2 +2H2O 2H + CO3 H2O + CO2 What do you notice about the number of atoms in the reactants and the products of the same chemical equation? What of you notice about the mass? 16 Appendix B3.1 - Action Law of Conservation of Mass Worksheet – Answer Key Instructions: * Fill out the reactants and products/ equations in the spaces provided. * Look at the reactants and in the space provided write out the number of atoms for each element. Do the same for the products. * Weigh the reactants on the scale and record the mass. Do the same for the products. Equation Reactants (R) Number of atoms in R Mass of R Products (P) Number of atoms in P Mass of P C2 + O2 + O2 --> CO2 + CO2 C2 + O2 + O2 O= 4 C= 2 N/A CO2 + CO2 O= 4 C= 2 N/A H2 + H2 + O2 --> H2O + H2O H2 + H2 + O2 H= 4 O= 2 N/A H2O + H2O H= 4 O= 2 N/A CH4 + 2O2 --> CO2 +2H2O CH4 + 2O2 C= 1 H= 4 O= 4 N/A CO2 + 2H2O C= 1 H= 4 O= 4 N/A 2H + CO3 --> H2O + CO2 2H + CO3 H= 2 O= 3 C= 1 N/A H2O + CO2 H= 2 O= 3 C= 1 N/A 6CO2 + 6H2O --> C6 H12 O6 + 6O2 C= 6 O= 18 H= 12 N/A C6 H12 O6 + 6O2 C= 6 O= 18 H= 12 N/A 6CO2 + 6H2O What do you notice about the number of atoms in the reactants and the products of the same chemical equation? What of you notice about the mass? The number of atoms in the reactants is the same as the number of atoms in the products. The mass of the reactants are equal to the mass of the products. 17 Appendix C1.0 – Consolidation & Connection Chemical Reactions that Affect our Environment Homework Sheet Combustion is an example of a chemical reaction that occurs abundantly on our planet. Unfortunately, there are many types of combustion reactions that negatively contribute to air pollution and greenhouse gas production. Combustion can occur in two forms: 1) Complete combustion - Occurs when the reactants burn completely in oxygen, and the products created are an oxide and water (H20) - An oxide can be created from many elements, but the most common oxide in combustion reactions is Carbon Dioxide (CO2), which is formed when any hydrocarbon is burned. The formula for a hydrocarbon being burned is as follows: CH4 + 2 O2 → CO2 + 2 H2O - Other common involved are Nitrogen, Sulphur and Iron, which also produce oxides. 2) Incomplete Combustion - Occurs when there is not enough oxygen to fully oxidize (burn) the reactants to produce CO2. - As a result, Carbon Monoxide (CO) is produced when a hydrocarbon is burned, along with CO2, H2O and H2 Ideally, all combustion reactions would be complete reactions, meaning that all reactants are fully oxidized and burned. However, the majority of these reactions that occur in our world are incomplete, resulting in other harmful products being created. As we can see, the burning of hydrocarbons can create dangerous and harmful greenhouse gases that are damaging to our environment. Some of the products created when these reactions happen are as follows: 1) 2) 3) 4) 5) 6) Ozone- causes smog and many healthy issues especially in the lungs. It also affects trees and plants, which are crucial to the life cycle Volatile Organic Compounds (VOCs) – these chemicals are cancer-causing agents. Nitrogen Dioxide (NO2) – causes smog and acid rain, and damages trees and buildings. Carbon Monoxide – causes a number of related health illnesses Chlorofluorocarbons (CFCs) – these damage the ozone layer and causes numerous health problems Carbon Dioxide (CO2) – a main ingredient in global warming, as it collects heat and light that is given off from the sun. Choose THREE of the six products produced from combustions reactions shown in the list above. For each of the THREE you have chosen: 1. Give an example of how they are released into our environment. 2. What are some measures our society can take to reduce the amount of these by-products released in our atmosphere? 3. What are some of the measures that YOU can take to reduce the amount of these by-products? 18 “Remember that life's A Great Balancing Act. And will you succeed? Yes! You will, indeed! (98 and ¾ percent guaranteed) Kid, you'll move mountains.” ― Dr. Seuss, Oh, the Places You'll Go! NAMES: Kalvin Lau & Holly Wykes UNIT: Chemical Reactions TITLE OF LESSON: Writing Balanced Chemical Equations BIG IDEAS: MATERIALS: - Chemicals react with each other in - Lego Bricks predictable ways - Weight Scale - Chemical reactions may have a negative - Balancing Chemical Equations Chart impact on the environment, but they can also be used to address environmental (1 per student) challenges. - Molecular Model Kits MINISTRY EXPECTATIONS: (1 per group of 4-5 students) - A1.12 Use appropriate numeric, symbolic, - Balancing Chemical Equations Activity and graphic modes of representation, and (1 per student) appropriate units of measurement (e.g., SI and imperial units) - A1.13 Express the results of calculations involving data accurately and precisely - C2.2 Construct molecular models to illustrate the structure of molecules in simple chemical reactions (e.g., C + O2 CO2; 2H2 + O2 2H2O), and produce diagrams of these models [PR, C] - C3.4 write word equations and balanced chemical equations for simple chemical reactions (e.g., 2H2 + O2 2H2O) STUDENT LEARNING GOALS: APPENDICES: 1. Understand basic definitions including A1.0 Minds-on Activity: Teaching Notes reactant, product, subscript and A1.1 Minds-on Activity: Chalkboard Notes coefficient. B1.0 Intro to Balancing Chemical Equations: 2. Understand the difference between a Teaching Notes skeleton chemical equation and a B1.1 Intro to Balancing Chemical Equations: balanced chemical equation. Student Handout 3. Construct, using molecular model kits, B1.2 Intro to Balancing Chemical Equations: reactants and products to create balanced Chalkboard Notes chemical equations. B2.0 Molecular Model Kits: Teaching Notes 4. Successfully complete the steps to balance B2.1 Molecular Model Kits: Chalkboard Notes written skeleton equations. B3.0 Balancing Chemical Equations Activity: 5. Apply knowledge of chemical equations to Teaching Notes & Solutions environmental challenges. B3.1 Balancing Chemical Equations Activity: Student Handout PRIOR KNOWLEDGE: C1.0 Oil Spill Application: Teaching Notes Definitions and examples regarding: Matter and C1.1 Oil Spill Application: Chalkboard Notes the Periodic Table; Ions, Molecules and Compounds; Chemical Reactions; Law of Conservation of Mass 19 A MINDS ON 5-10 mins B ACTION 20-25 mins 15-20 mins 15-20 mins C CONSOLIDATION & CONNECTION T/L STRATEGIES: 1) Conservation of Mass Demo Show sample of Lego bricks. Demonstrate how pieces combine to create a new structure. How does this relate to Conservation of Mass? Appendix A1.0 1) Introduction to Balancing Chemical Equations Chart Give example skeleton equation and show how to balance using coefficients and Law of Conservation of Mass. Fill in chalkboard chart showing steps to balancing a skeleton chemical equation. Appendix B1.0; B1.1; B1.2 2) Molecular Model Construction: In groups of 4-5, students construct reactants and products using Molecular Model kits and demonstrate correctly balanced models. Appendix B2.0; B2.1 3) Balancing Chemical Equations Activity: Independently, students complete a problem-solving handout quizzing definitions and correct balancing of chemical equations. Appendix B3.0; B3.1 1) Oil Spill Clean-up Application: Discussion of how chemicals can be added to oil spills to neutralize toxicity in environment. RATIONALE: Allow students to recall the topic and think about applications of Conservation of Mass. ASSESSMENT: Predict/Observe/ Explain, Think/pair/share. Introduce concept of balancing chemical equations using coefficients and demonstrate correct method of solving skeleton equations using chart on chalkboard. Allows students to visualize skeleton and balanced chemical equations, provide practice of balancing chemical equations using 3-dimensional models. Participation in discussion, correct answers to fill out chalkboard chart. Provides individual practice of basic definitions and problem solving regarding balancing chemical equations. Correct answers as a group, taken up in class to consolidate learning. Applies application and analysis of learned concepts to real-life examples, solidify importance of balanced chemical equations. Think/pair/share, discussion format in class, contributions to filling out chart. Presentation of correctly balanced chemical equations, explanation to classmates, filling in correct coefficients on chalkboard. 5-10 mins NEXT STEPS Appendix C1.0; C1.1 - Strategies for Balancing Chemical Equations - Chapter Quiz - Introduction to Acids and Bases (next unit) 20 Appendix A1.0 Minds-on Activity – Teaching Notes - Purpose: demonstrate the law of conservation of mass. Display of scale and unassembled Lego model at front of class. Unassembled bricks represent reactants and the assembled model will represent the products – items can be substituted with other materials of similar concept e.g. puzzle Questions to ask: “We have Lego bricks here, how much do you think they weigh? Let’s see how much they weigh.” Weigh bricks and record in table Predict: “After we assemble the model, do you think it will weigh more or less than the parts?” Observe: Weigh the assembled model and record in table “What if we had enough parts to make a few cars? Would the parts weigh more than the assembled cars?” Weight two sets of unassembled bricks. Record in table. Assemble the bricks and weight again. Record in table. Explain: “What did you notice? Why does this happen?” Weigh reactants and then assemble model and weight product. Demonstrate they are the same weight. Review the law of conservation of mass The mass of substances produced (products) by a chemical reaction is always equal to the mass of the reacting substances (reactants) 21 Appendix A1.1 Minds-on Activity – Chalkboard Notes Weight of items (g) Unassembled bricks Assembled bricks 1set 2 sets 22 Appendix B1.0 Introduction to Balancing Chemical Equations – Teaching Notes - The rearrangement of atoms that occurs during a chemical reaction can be illustrated using models or diagrams. - E.g. Hydrogen + Oxygen Water - To balance a chemical reaction, we need to apply the Law of Conservation of Mass: In a chemical reaction, the mass of the products always equals the mass of the reactants. - In the formula for this reaction, there has to be equal numbers of hydrogen atoms and oxygen atoms on both the reactants and products side. - When the number of each kind of atom is the same in reactants and products, the equation is said to be balanced. - The equation on the board is a skeleton equation; that is, the names of the elements are correct, but the numbers of each differ on the reactants and products sides of the equation. - To reach the correct number of elements on each side of the equation, add coefficients to the reactants and products to change the ratio of each within the equation. - Note that the subscripts do not change as these indicate the ratio of elements within each formula. - E.g. 2H2 + O2 2H2O H2 + O2 H2O 23 Teaching Notes (Cont’d) – Filling in the Chart Balance the following chemical equation: AlBr3 + Cl2 AlCl3 + Br2 1. Count the number of atoms of each element in the skeleton equation. Reactants AlBr3 Cl2 Number of Atoms Al = 1 Br = 3 Cl = 2 Products AlCl3 Br2 Number of Atoms Al = 1 Cl = 3 Br = 2 2. Balance the number of bromine atoms by adding a coefficient of 2 in front of AlBr3 and a coefficient of 3 in front of Br2. Count the number of atoms again by multiplying the coefficient by the number of atoms in the formula. Reactants 2AlBr3 Cl2 Number of Atoms Al = 2 x 1 = 2 Br = 2 x 3 = 6 Cl = 2 Products AlCl3 3Br2 Number of Atoms Al = 1 Cl = 3 Br = 2 3. Balance the number of aluminum atoms by adding a coefficient of 2 in front of AlCl3. Count the atoms again. Reactants 2AlBr3 Cl2 Number of Atoms Al = 2 x 1 = 2 Br = 2 x 3 = 6 Cl = 2 Products 2AlCl3 3Br2 Number of Atoms Al = 2 x 1 = 2 Cl = 2 x 3 = 6 Br = 3 x 2 = 6 4. Balance the number of chlorine atoms by adding a coefficient of 3 in front of Cl2. Count the atoms again. Reactants 2AlBr3 3Cl2 Number of Atoms Al = 2 x 1 = 2 Br = 2 x 3 = 6 Cl = 3 x 2 = 6 Products 2AlCl3 3Br2 Number of Atoms Al = 2 x 1 = 2 Cl = 2 x 3 = 6 Br = 3 x 2 = 6 The equation is now balanced: 2AlBr3 + 3Cl2 2AlCl3 + 3Br2 24 Appendix B1.1 Introduction to Balancing Chemical Equations – Chalkboard notes Chalkboard 1 – Steps 1. Count element atoms in both reactants and products. 2. Select an element to balance that is not hydrogen or oxygen, e.g. Bromine. Add coefficients to compounds and elements that contain Bromine and then count atoms again. 3. Balance the next element by adding coefficients to compounds containing element, e.g. Aluminum and count atoms again. 4. Balance next element that is not balanced yet, e.g. Chlorine. Add coefficients to compounds or elements that contain Chlorine. Count the number of element atoms. 5. Continue process until all elements are balanced. Once all elements are balanced, the equation is complete. Recount the atoms to double check your work. Chalkboard 2 – Chart Skeleton Equation: AlBr3 + Cl2 AlCl3 + Br2 Reactants AlBr3 Cl2 Number of Atoms Products AlCl3 Number of Atoms Br2 25 Appendix B1.2 Introduction to Balancing Chemical Equations – Student Handout Balance the following chemical equation: AlBr3 + Cl2 AlCl3 + Br2 1. Count the number of atoms of each element in the skeleton equation. Reactants Number of Atoms Products Number of Atoms 2. Balance the number of bromine atoms by adding a coefficient of 2 in front of AlBr3 and a coefficient of 3 in front of Br2. Count the number of atoms again by multiplying the coefficient by the number of atoms in the formula. Reactants Number of Atoms Products Number of Atoms 3. Balance the number of aluminum atoms by adding a coefficient of 2 in front of AlCl3. Count the atoms again. Reactants Number of Atoms Products Number of Atoms 4. Balance the number of chlorine atoms by adding a coefficient of 3 in front of Cl2. Count the atoms again. Reactants Number of Atoms Products Number of Atoms 26 Appendix 2.0 Molecular Model Kits – Teaching Notes 1. Divide class into groups of 4-5. 2. Provide each group with one Molecular Model Kit. 3. Ask each group to construct one of the following skeleton equations: a. b. c. d. e. H2 + O2 H2O Na + O2 Na2O2 CO2 + H2O H2CO3 N2 + H2 NH3 Fe + Cl2 FeCl3 4. Ask each group to balance the constructed skeleton equations by building additional molecules as needed. 5. A volunteer from each group will present their findings in molecular model form. 6. A volunteer from each group will fill in any coefficients needed to balance the skeleton equations as written on the blackboard. 27 Appendix B2.1 Molecular Model Kits – Chalkboard Notes a. 2H2 + O2 2H2O b. 2Na + O2 Na2O2 c. 2CO2 + 2H2O 2H2CO3 d. N2 + 3H2 2NH3 e. 2Fe + 3Cl2 2FeCl3 28 Appendix B3.0 Balancing Chemical Equations Activity – Teaching Notes 1. Give each student one handout face down on the desks. 2. Ask each student to work independently to answer the questions to the best of their ability. 3. Be sure to erase the chalkboard notes! 4. Allow 15-20 minutes to complete the activity. Answer Key: 1. A skeleton equation is an equation showing the reactants and products in their molecular state. A balanced equation uses coefficients to apply the Law of Conservation of Mass to the equations and contains the same number of atoms on both sides of the equation. 2. A subscript gives the ratio of elements in a formula; they cannot change in a given chemical. A coefficient gives the ratio of the reactants and products in the reaction; thus are used to balance chemical equations. 3. a. b. c. d. e. f. 2H2 + O2 2H2O 4Na + O2 2Na2O2 2CO2 + 2H2O 2H2CO3 N2 + 3H2 2NH3 2Fe + 3Cl2 2FeCl3 CH4 + 2O2 CO2 + 2H2O 4. a. 2C8H18 + 25O2 16CO2 + 18H2O b. 25 carbon dioxide molecules 29 Appendix B3.1 Balancing Chemical Equations Activity – Student Handout 1. What is the difference between a skeleton equation and a balanced equation? 2. What is the difference between a subscript and a coefficient in a chemical equation? 3. Balance the following skeleton equations: a. H2 + O2 H2O b. Na + O2 Na2O2 c. CO2 + H2O H2CO3 d. N2 + H2 NH3 e. Fe + Cl2 FeCl3 f. CH4 + O2 CO2 + H2O 4. Octane, C8H18, is a compound in gasoline. Octane burns in oxygen to produce carbon dioxide gas and water vapour. a. Write a balanced chemical equation for this reaction. b. How many carbon dioxide molecules are produced for every molecule of octane burned? 30 Appendix C1.0 Oil Spill Application – Teaching Notes Introduce the concept of environmental application for the use of balancing chemical equations. One of the ways in which environmental workers clean up oil spills is by adding another chemical to the spill to neutralize the toxicity of the pollutants. Using the knowledge obtained regarding the Law of Conservation of Mass and balancing chemical equations: 1. What might happen if too little neutralizing chemical is added to an oil spill? 2. What might happen if too much neutralizing chemical is added to an oil spill? 3. What could be the effects of an oil spill that is not attended to? To be completed in a Think/Pair/Share format. Ask students to write their responses in the chart. 31 Appendix C1.1 Oil Spill Application – Chalkboard Notes Too Little Chemical More reactant (oil) than product (neutral substance) - Not all oil is neutralized, toxicity remains - Too Much Chemical More reactant (neutralizing substance) remains - Neutralizing substance could pose additional environmental threat i.e. if toxic on its own - Unattended to Spill Toxicity may affect plant, animal, aquatic life - Altered pH of water where oil is spilled - 32





