Molecular Structure & Bonding: H2, Hybridization, VSEPR
advertisement
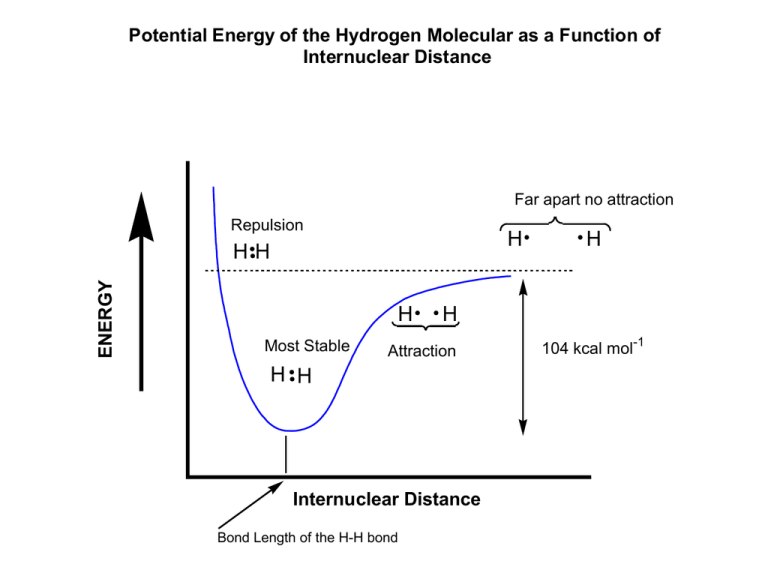
Potential Energy of the Hydrogen Molecular as a Function of Internuclear Distance Far apart no attraction Repulsion H ENERGY HH H Most Stable H Attraction H H Internuclear Distance Bond Length of the H-H bond H 104 kcal mol-1 Pictorial Representation of Bonding and Antibonding Orbitals for the Hydrogen Molecule Anti-bonding Orbital addition of orbitals with opposite phases Bonding Orbital addition of orbitals with the same phase Wave Characteristics of the Electron Planar Wave Has Positive and Negative Phases + - + + - - Electron has wave characteristics and this includes positive and negative phases Do not confuse the phase with the charge of the electron! Overlaping Waves of the same phase reinforce + + + Overlaping Waves of opposite phase produce a node (zero applitude) node Energy Diagram For the Hydrogen Molecule ENERGY Antibonding Molecular Orbital Atomic Orbital Energy of the isolated hydrogen atom Atomic Orbital Bonding Molecular Orbital Energy of the isolated hydrogen atom Mixing of Atomic Orbitals to Make Hybrid Orbitals Mix 1 2s with 3 2p orbitals get 3 4 sp orbitals 25% s character 75% p character Overlap of a sp3 hybrid orbital with a 1s orbital to make a sigma C-H bond. Methane has 4 C-H sigma bonds. Four bonds of equal length. Bond vector separated by 109.5°. This angle also happens to be the angle that places the substituents around the carbon as far apart as possible. Since each electron pair of the 4 sigma bonds will be concentrated between the C-H atoms, the bond angle minimizes the repulsion between the electron pair of one bond and its 3 neighbouring bonds H 109.5º H H C H bond length H H H bond angle C H Structure of Ethane C2H6 Lewis or Dot Structure H H H C C H H H H H H Line Bond Structure H H H C C H H H H C C H H Sigma bond caused by overlap of two sp3 orbitals C C Hybridization: The structure of Ethene or Ethylene - sp2 Hybrid Orbitals Ethylene C2H4 Since Carbon is tetravalent ethylene must contain a carbon to carbon double bond. H H C H H H C C H Trivalent carbon not allowed H H H C C H H C H Tetravalent carbon Line Bond Structure Lewis Structure sp2 Hybridization Viewed from above Viewed from side unhybridized p orbital 120º 120º C C 120º sp2 hybrid orbitals Ethylene C2H4 H H C C H H 2 Overlap of 2 sp orbitals gives C-C sigma bond H H C H H H C C H H C H sp Hybridization:- Acetylene C2H2 C C H H Overlap of 2 sp orbitals gives C-C sigma bond H C C H H C C H Predicting Shapes of Molecules Valence-Shell Electron Pair Repulsion VSEPR Model - electrons in a molecule arrange themselves to minimize electrostatic repulsion. CCl4 like methane (CH4) is tetrahedral because this is how the four groups of electron pairs that make the bonds between carbon and chlorine can be the farthest apart. Valence-Shell Electron Pair Repulsion VSEPR To Apply this idea use the following steps:1. Count electron groups on an atom. These are of the following type Lone Pair - one group Any pair or set of pairs of electrons - one group. 2. Assume each group moves as far apart as possible. Acetylene C2H2 The geometry of the molecule is linear and the bond angle = 180°. H C C H H Two groups of electrons one of two and one of 6 electrons. Molecule is linear C C H CH5N H H Geometry around carbon C N H H H Geometry around nitrogen ? CH5N H H Geometry around carbon C N H H H Geometry around nitrogen ? The carbon atom is surrounded by three hydrogen atoms and one N. The Nitrogen has a lone pair is attached to one carbon and two hydrogens. Therefore, we would predict that the carbon would have tetrahedral geometry and is sp3 hybridized, the same for nitrogen. C2H6O There are two plausible Lewis or Line Bond structures. Both are acceptable structures. H3C O CH3 H3C CH2 O H C2H6O There are two plausible Lewis or Line Bond structures. Both are acceptable structures. H3C O CH3 The carbon atom is surrounded by three hydrogen atoms and one O. The Oxygen has two lone pairs and is attached to two carbons. Therefore, we would predict that the carbon would have tetrahedral geometry and is sp3 hybridized, the same for oxygen with two lone pairs and two bonds to different carbon atoms. H3C CH2 O H The carbon atoms all surrounded by 4 groups . The Oxygen has two lone pairs and is attached to one carbon and one hydrogen atom. Therefore, we would predict that the carbon atoms would have tetrahedral geometry and are sp3 hybridized, the same for oxygen with two lone pairs and two bonds to different carbon atoms. Benzene C6H6 H H H H H H Geometry at this carbon? Benzene C6H6 H H H H H Geometry at this carbon? H Each carbon has an identical set of groups surround it. one hydrogen one C-C double bond and one C-C single bond for a total of 3 groups. Therefore, we would predict 2 that each carbon would be planar and sp hybridized Acetonitrile C2H3N Lewis structure? Hybridization? Shape?