CERAMICS
advertisement

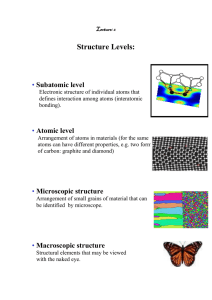
King Fahd University of Petroleum & Minerals Mechanical Engineering Department ME 205 – 01&02 : MATERIALS SCIENCE Fall Semester 2006-2007 (061) Instructor: Mr. Muhammad Younas Office: 22-206 Phone: 3049 Office Hours: SMW ( 9:00-9:50 AM ) & UT ( 11:00-11:50 AM ) E-mail: myounasa@kfupm.edu.sa Textbook Callister, W.D., Materials Science and Engineering 6th Ed., 2003 Lecture Schedule Lecture # Lecture Topic Section# 1 Classification of Materials, Materials of Future 1.4, 1.6 2 The Periodic Table, Bonding Forces and Energies. 2.4,2.5 3 Primary and Secondary Bonds. 2.6, 2.7 4 Crystal Structures, Unit Cells. 3.2,3.3 5 Metallic Crystal Structures, Density Computation. 3.4,3.5 6 Polymorphism, Crystal systems, Point coordinates 3.6,3.7, 3.8 7 Crystallographic Directions, Crystallographic Planes. 3.9, 3.10 8 Linear and Planar Atomic Densities. 3.11 9 Closed Packed Crystal Structures, Single Crystals, Polycrystalline Materials, Anisotropy. 3.12, 3.13, 3.14,3.15 10 Imperfection in Solids, Point Defects, Vacancies and Self-interstitials, Impurities in Solids. Specification of composition 4.2, 4.3, 4.4 11 Dislocations-Linear Defects, Interfacial Defects (external surface & grain boundaries only). 4.5, 4.6 EXAM 1: Sunday October 29, 2006 @ 7:00 – 9:00 PM 12 Diffusion, Introduction, Diffusion Mechanisms. 5.1,5.2 13 Steady-State Diffusion. 5.3 14 Non-Steady State Diffusion. Factors that influence Diffusion. 5.4, 5.5 15 Mechanical Properties of Metals, Elastic Deformation, Concepts of Stress and Strain. 6.2 16 Stress-Strain Behavior, Elastic Properties of Materials. 6.3, 6.5 17 Plastic Deformation, Tensile Properties. 6.6 18 Tensile Properties 6.6 19 True Stress-True Strain, Elastic Recovery During Plastic Deformation. 6.7, 6.8 20 Dislocations and Strengthening Mechanisms, Basic concepts, Characteristics of Dislocations. 7.2, 7.3 21 Slip Systems. 7.4 22 Slip in Single Crystals, Plastic Deformation of Polycrystalline Materials. 7.5, 7.6 Lecture # Lecture Topic Section# 23 Strengthening by Grain Size Reduction, Solid Solution Strengthening, Strain Hardening. 7.8, 7.9, 7.10 24 Recovery, Recrystallization and Grain growth. 7.11, 7.12, 7.13 EXAM 2 November 29, 2006 @ 5:30 – 7:30 PM 25 Phase Diagram, Solubility Limit, Phases, Microstructure, Phase Equilibria. 9.1, 9.2, 9.3, 9.4, 9.5 26 Binary Iso-morphous System 9.6,9.7 27 Interpretation of Phase diagram 9.7, 9.8 28 Binary Eutectic Phase Diagrams, Development of Microstructure in Eutectic Alloys 9.10, 9.11 29 Iron-Iron Carbide Phase Diagram, Development of Microstructure in in IronCarbon Alloys, The influence of other alloying elements 9.17, 9.18, 9.18 30 Review class Final Exam Grading Policy 1) Home works : 05% 2) Quizzes : 10% 3) Lab. Work : 15% 4) Exam # 1 : 15% 5) Exam # 2 : 20% 6) Final Exam : 35% CLASS ATTENDANCE Attendance in the class will be strictly observed starting first day of classes. IN CASE OF AN UNEXCUSED ABSENCE, 0.5 POINT WILL BE DEDUCTED FROM FINAL GRADE. A DN grade will be immediately reported for SIX (6) unexcused absences. A DN grade will be immediately reported if both unexcused and excused absences reach TEN (10) absences. Materials Science and Engineering Material Science Involves investigating the relationships that exist between the structures and properties of materials. Materials Engineering On the basis of structure-property correlations, involves designing or engineering the structure of a material to produce a predetermined set of properties. Materials Science and Engineering (Contd.) The structure of a material usually relates to the arrangement of its internal components. Subatomic structure involves electrons within the individual atoms and interactions with their nuclei. On an atomic level, structure encompasses the organization of atoms or molecules relative to one another. Microscopic structure contains large groups of atoms that are normally agglomerated together and subject to direct observation using some type of microscope. Macroscopic structure meaning structural elements that may be viewed with naked eye. Materials Science and Engineering (Contd.) 1. 2. 3. 4. 5. 6. Property is a material trait in terms of the kind and magnitude of response to a specified imposed stimulus. It is independent of shape and size. Six categories of material properties: Mechanical properties relate deformation to an applied load or force; examples include elastic modulus and strength. For electrical properties, such as electrical conductivity and dielectric constant, the stimulus is an electric field. The thermal behavior can be represented in terms of heat capacity and thermal conductivity. Magnetic properties demonstrate the response of a material to the application of a magnetic field. For optical properties, the stimulus is electromagnetic or light radiation; index of refraction and reflectivity are representative optical properties. Deteriorative characteristics indicate the chemical reactivity of materials. Materials Science and Engineering (Contd.) Four components involved in the science and engineering of materials, and their interrelationship: Processing ===> Structure ===> Properties ===> Performance CLASSIFICATION OF MATERIALS Major Classes Of Materials • 1. • 2. • 3. • 4. • 5. • 6. METALS CERAMICS POLYMERS COMPOSITES ELECTRONIC MATERIALS BIO MATERIALS BASIS OF MATERIAL CLASSIFICATIONS Chemical Makeup Atomic Bonding Atomic Arrangement Characteristic Physical Properties Characteristic Mechanical Properties METALS Distinguishing o o o o o o o Features Atoms arranged in a regular repeating manner. Relatively High Strength. High Density. Ductile. Excellent conductors of Electricity and Heat. Opaque to visible light. Shiny appearance. APPLICATIONS OF METALS Electrical wiring Buildings, Structures, Bridges etc. Automobiles: body, chassis, engine block, springs, etc. Air planes: engines, fuselage (airplane body), landing gears, etc. Trains: rails, engines, body, wheels Machines Machine tools: drills, hammers, saw blades, nuts, bolts, etc. Industrial Plant components, structures Magnets METALLIC MATERIAL EXAMPLES Pure Metals Cu, Fe, Zn, Al, Ag, Au, Cr, Ni, Sn, etc Alloys Steel, Brass, Stainless Steels, etc. CERAMICS Distinguishing Features Most have a regular arrangement of atoms (except glasses) Compounds of Metallic and NonMetallic elements Density lower than Metals Stronger than Metals Low resistance to Fracture Brittle (low ductility) High Melting Points Poor Conductors of Electricity and Heat APPLICATIONS OF CERAMICS Electrical Insulators Thermal Insulations and Coatings Abrasives Glasses (windows, TV screens, Optical fibers Cement, Concrete Ceramic tiles for space shuttles Furnace Lining bricks CERAMIC MATERIAL EXAMPLES Diamond, Graphite Glasses Building Materials Oxides (SiO2, Al2O3) Carbide Tools (WC, TiC) POLYMERS Distinguishing Feature Composed Primarily of C and H (hydrocarbons) Low Melting Points Some partly crystalline, Most are not Most are poor conductors of Electricity and Heat Many have high plasticity Some are transparent, most are opaque APPLICATIONS OF POLYMERS Adhesives and Glues Plastic products (plastic pipes, bottles, house hold utensils, etc.) Coatings and Paints Solid Lubricants (Teflon) Rubber Products (gaskets, seals, and orings) Clothing and furniture coverings (leather, nylon) EXAMPLES OF POLYMER MATERIALS PVC (Poly Vinyl Chlorides) PE (Poly ethylene) PC (Poly Carbonates) Teflon Nylon COMPOSITES Distinguishing Features Composed of Two or More Different Materials Strong, Light weight, Good resistance to fracture High stiffness and good deformability Collection of good Properties of each material used APPLICATIONS OF COMPOSITE MATERIALS Aerospace, Marine, Automotive Sporting Goods Storage Tanks (water, fuel, chemicals) Transport Piping (oil, seawater, sewage) EXAMPLES OF COMPOSITE MATERIALS PMCs (polymer matrix composites) Fiber Glass, Concrete) MMCs (Metal Matrix Composites) CMCs (Ceramic Matrix Composites) The bridge in the picture is built entirely from composite material. Weighs one-tenth of the conventional concrete bridge. It took only 18 hours to assemble the bridge. MATERIALS OF FUTURE SMART MATERIALS Shape Memory Alloys Piezoelectric ceramics MEMS (Micro-Electrical Mechanical Systems) NANOTECHNOLOGY Materials by design Carbon Nanotubes (500 atom diameters)