OBJ 4
advertisement

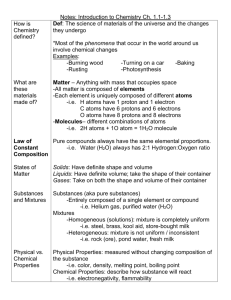
OBJ 4 (Chemistry) Question 1 • Upon entering your lab station, you realize you have more chemicals than you need in order to complete your assignment. What should you do? Question 1 • Upon entering your lab station, you realize you have more chemicals than you need in order to complete your assignment. What should you do? • Use the proper amount of chemicals called for and return any unused chemicals to the teacher. Question 2 • You are instructed to find the density of a small, irregularly shaped item. Which set of equipment is best for completing this assignment? Question 2 • You are instructed to find the density of a small, irregularly shaped item. Which set of equipment is best for completing this assignment? • a graduated cylinder and triple beam balance Question 3 • How much water is in the graduated cylinder? Question 3 • How much water is in the graduated cylinder? 40.7 ml Question 4 • What is the charge, location, and relative size of a proton? Question 4 • What is the charge, location, and relative size of a proton? • Positive (+), in the nucleus, and about the size of a neutron. Question 5 • The above graph shows the data collected when a solid was melted. At which point on the graph had the solid turned into a liquid? Question 5 • The above graph shows the data collected when a solid was melted. At which point on the graph had the solid turned into a liquid? • C Question 6 • During a laboratory experiment, two substances are combined in a dish. At the end of the experiment, you use filtration to retrieve both original substances. What was made? Question 6 • During a laboratory experiment, two substances are combined in a dish. At the end of the experiment, you use filtration to retrieve both original substances. Determine the type of product formed when these two substances were combined? • MIXTURE Question 7 • Before he began an experiment, Chris wrote out the steps to his procedure and recorded his conclusion. During the experiment, he noticed an error in the steps, and adjusted them to get to the conclusion he predicted. What did he DO WRONG? Question 7 • Before he began an experiment, Chris wrote out the steps to his procedure and recorded his conclusion. During the experiment, he noticed an error in the steps, and adjusted them to get to the conclusion he predicted. What did he DO WRONG? • No hypothesis……. Question 8 • You want to buy a heating system for your backyard pool. However, you want to make sure that the energy source of this heating system does not produce any greenhouse gases. What type of energy system can be used without any production of greenhouse gases? Question 8 • You want to buy a heating system for your backyard pool. However, you want to make sure that the energy source of this heating system does not produce any greenhouse gases. What type of energy system can be used without any production of greenhouse gases? • SOLAR Question 9 • Hybrid cars are a combination of research done on which two common car parts? Question 9 • Hybrid cars are a combination of research done on which two common car parts? • engines and batteries Question 10 • Agricultural scientists perform a very useful job by making sure that fertilizers and pesticides are used within safe levels for humans and the environment. Which of the following areas of study would be useful in determining the levels of these compounds? Question 10 • Agricultural scientists perform a very useful job by making sure that fertilizers and pesticides are used within safe levels for humans and the environment. Which of the following areas of study would be useful in determining the levels of these compounds? • CHEMISTRY Question 11 • Alchemists in the early 1100's tried to convert common metals into gold. They developed experimental procedures and apparatus to gain knowledge about the characteristics of substances. What is the best way to describe an alchemist in modern terms? Question 11 • Alchemists in the early 1100's tried to convert common metals into gold. They developed experimental procedures and apparatus to gain knowledge about the characteristics of substances. What is the best way to describe an alchemist in modern terms? • SCIENTIST Question 12 • Lisa has two rings with the same size and diameter. One is made of platinum, and the other is made of gold. What aspect of platinum makes that ring heavier than the gold ring? Question 12 • Lisa has two rings with the same size and diameter. One is made of platinum, and the other is made of gold. What aspect of platinum makes that ring heavier than the gold ring? • Platinum is more dense than gold. Question 13 • Silicon dioxide (SiO2), also called silica, occurs naturally in many different forms that have different properties, such as quartz, sand, and Cristobalite. What is the primary reason for the difference in properties (hardness, brittleness, boiling point, etc.) between these forms? Question 13 • Silicon dioxide (SiO2), also called silica, occurs naturally in many different forms that have different properties, such as quartz, sand, and Cristobalite. What is the primary reason for the difference in properties (hardness, brittleness, boiling point, etc.) between these forms? • differences in intermolecular forces Question 14 • A student was given the following list of properties of an unknown substance: Its molecules exhibit properties of its constituent atoms. It exists as a gas in its free state. Its molecules are diatomic. It is a pure substance. Which indicates that it is an element? Question 14 • A student was given the following list of properties of an unknown substance: Its molecules exhibit properties of its constituent atoms. It exists as a gas in its free state. Its molecules are diatomic. It is a pure substance. Which indicates that it is an element? Its molecules exhibit properties of its constituent atoms. Question 15 • The periodic table divides elements into groups and periods. Which group or period contains atoms that are all radioactive? Question 15 • The periodic table divides elements into groups and periods. Which group or period contains atoms that are all radioactive? • actinides Question 16 • Which of the following indicates an increase in the potential energy of molecules in a unit of matter? Question 16 • Which of the following indicates an increase in the potential energy of molecules in a unit of matter? • The matter changes from the solid to the liquid phase. Question 17 • When an ax is swung, it has kinetic energy. When the ax connects with a piece of wood, the kinetic energy causes the movement of the ax into the wood and the wood splits. What other type of energy is produced when the ax connects with the wood? Question 17 • When an ax is swung, it has kinetic energy. When the ax connects with a piece of wood, the kinetic energy causes the movement of the ax into the wood and the wood splits. What other type of energy is produced when the ax connects with the wood? heat energy Question 18 • What is the charge, location, and size of electrons? Question 18 • What is the charge, location, and size of electrons? • Electrons are negative and are located outside the nucleus……and are the smallest Question 19 • Carbon has multiple isotopes. Two naturally occurring isotopes of carbon are Carbon - 14 and Carbon -12. What aspect of Carbon -14 makes it useful for carbon dating? Question 19 • Carbon has multiple isotopes. Two naturally occurring isotopes of carbon are Carbon - 14 and Carbon -12. What aspect of Carbon -14 makes it useful for carbon dating? • It has a long half-life Question 20 • If the volume of a sample of ideal gas is doubled at constant temperature, the pressure of the gas will— Question 20 • If the volume of a sample of ideal gas is doubled at constant temperature, the pressure of the gas will— • be half the initial pressure. Question 21 • During an investigation with gases, you blow up a balloon and tie it off. Your teacher has you place the balloon in a freezer overnight. The air pressure inside the freezer remains constant. What can you predict about the balloon when you remove it from the freezer? Question 21 • During an investigation with gases, you blow up a balloon and tie it off. Your teacher has you place the balloon in a freezer overnight. The air pressure inside the freezer remains constant. What can you predict about the balloon when you remove it from the freezer? • The volume of the balloon will have decreased. Question 22 • When methane (CH4) is formed, one carbon atom is combined with four hydrogen atoms. Why are more hydrogen atoms than carbon atoms needed to form this compound? Question 22 • When methane (CH4) is formed, one carbon atom is combined with four hydrogen atoms. Why are more hydrogen atoms than carbon atoms needed to form this compound? • Carbon needs more electrons. Question 23 • Which of the following is a property associated with ionic compounds? Question 23 • Which of the following is a property associated with ionic compounds? • An ionic compound is usually composed of a nonmetal and a metal. Question 24 • In which of the following substances does the repulsion between electron pairs cause the bonds to adjust to keep the valence electron pairs as far apart as possible in the arrangement of atoms? Question 24 • In which of the following substances does the repulsion between electron pairs cause the bonds to adjust to keep the valence electron pairs as far apart as possible in the arrangement of atoms? • molecules Question 25 • Methane (CH4) is a highly flammable natural gas. What property of this gas causes its flammability? Question 25 • Methane (CH4) is a highly flammable natural gas. What property of this gas causes its flammability? • Methane is a hydrocarbon. Question 26 • Energy is produced in the Sun by a nuclear fusion reaction in which two hydrogen atoms combine to form a helium atom. Two hydrogen atoms can also react to form hydrogen gas (H2). What best explains why the fusion reaction involves a great change in energy but the hydrogen gas reaction involves only a small change in energy? Question 26 • Energy is produced in the Sun by a nuclear fusion reaction in which two hydrogen atoms combine to form a helium atom. Two hydrogen atoms can also react to form hydrogen gas (H2). What best explains why the fusion reaction involves a great change in energy but the hydrogen gas reaction involves only a small change in energy? • The fusion reaction has nuclear forces as the energy source. Question 27 • How much of a sample of a radioactive element will remain after three half-lives? Question 27 • How much of a sample of a radioactive element will remain after three half-lives? • Only 1/8th of the sample will remain • 1st half life = 50% • 2nd half-life = 25% • 3rd half –life = 12.50% = 1/8 Question 28 • The radioisotope technetium-99 is commonly used in nuclear medicine on human beings. It has a half life of six hours, and emits gamma rays that are detected using a camera. What makes it possible for this radioactive material to be used internally in a human body? Question 28 • The radioisotope technetium-99 is commonly used in nuclear medicine on human beings. It has a half life of six hours, and emits gamma rays that are detected using a camera. What makes it possible for this radioactive material to be used internally in a human body? • It has a short half-life. Question 29 • A worker at a nuclear power plant discovers a crack in one of the used fuel cell storage pools. What hazards does this create? Question 29 • A worker at a nuclear power plant discovers a crack in one of the used fuel cell storage pools. What hazards does this create? • radiation leakages into the soil Question 30 • What is an element that can oxidize during a chemical reaction. Question 30 • What can oxidize during a chemical reaction? • magnesium Question 30 • What can oxidize during a chemical reaction? • magnesium Question 31 • What is the name of the compound represented by N2F4? Question 31 • What is the name of the compound represented by N2F4? • dinitrogen tetrafluoride • di = 2 tetra = 4 Question 32 • What is the molecular formula of tetraphosphorus decoxide? Question 32 • What is the molecular formula of tetraphosphorus decoxide? • P4O10 • Tetra = 4 Deca = 10 Question 33 • ___NH3 + ___O2 → ___N2 + ___H2O If the equation above were balanced with lowest-whole-number coefficients, the coefficient for NH3 would be Question 33 • ___NH3 + ___O2 → ___N2 + ___H2O If the equation above were balanced with lowest-whole-number coefficients, the coefficient for NH3 would be • 4 Question 33 • ___NH3 + ___O2 → ___N2 + ___H2O If the equation above were balanced with lowest-whole-number coefficients, the coefficient for NH3 would be Question 34 • Ben is conducting an experiment. Ben places 5 grams of sugar in 100 milliliters of water. Ben measures how quickly the sugar dissolves. Ben takes another 5 gram sample of sugar. Ben grinds the sugar particles into fine granules. Ben places 5 grams of fine sugar granules into 100 milliliters of water. Ben measures how quickly the sugar dissolves. What is the experiment testing? Question 34 • Ben is conducting an experiment. Ben places 5 grams of sugar in 100 milliliters of water. Ben measures how quickly the sugar dissolves. Ben takes another 5 gram sample of sugar. Ben grinds the sugar particles into fine granules. Ben places 5 grams of fine sugar granules into 100 milliliters of water. Ben measures how quickly the sugar dissolves. What is the experiment testing? • HOW PARTICLE SIZE AFFECTS SOLUBILITY Question 35 • What will not increase the dissolving rate for a solvent? Question 35 • What will not increase the dissolving rate for a solvent? • increasing the amount of solute Question 36 • A specific liquid is known as the universal solvent, because it is important as a solvent not only in living organisms but also in the environment. Identify this liquid. Question 36 • A specific liquid is known as the universal solvent, because it is important as a solvent not only in living organisms but also in the environment. Identify this liquid. • water Question 37 • Rosa needs to make a supersaturated solution for an experiment. What is the BEST way Rosa can make a supersaturated solution? Question 37 • Rosa needs to make a supersaturated solution for an experiment. What is the BEST way Rosa can make a supersaturated solution? • 77 • Raise the temperature of the solvent while adding the solute. Question 38 • Regina performed two experiments. The first experiment involved one gram of salt dissolved in 200 milliliters of water. Her second experiment involved one gram of salt dissolved in 1 liter of water. In which experiment did the solute dissolve faster? Question 38 • Regina performed two experiments. The first experiment involved one gram of salt dissolved in 200 milliliters of water. Her second experiment involved one gram of salt dissolved in 1 liter of water. In which experiment did the solute dissolve faster? • The salt dissolved faster in the 1 L of water Question 39 • Drain cleaners commonly used at home contain NaOH (sodium hydroxide). This turns red litmus paper blue. What is the nature of these cleaners? Question 39 • Drain cleaners commonly used at home contain NaOH (sodium hydroxide). This turns red litmus paper blue. What is the nature of these cleaners? • Basic (They are bases) Question 40 • Determine which of the following substances will not conduct electricity well when it is dissolved in water. Question 40 • Determine which of the following substances will not conduct electricity well when it is dissolved in water. • covalent compounds Question 41 • The pH of swimming pool water is maintained at acidic levels to prevent the growth of algae and bacteria. What conclusion can be drawn from this information? Question 41 • The pH of swimming pool water is maintained at acidic levels to prevent the growth of algae and bacteria. What conclusion can be drawn from this information? • Acidic water is harmful for the aquatic life in natural systems.