11sep19.ppt
advertisement
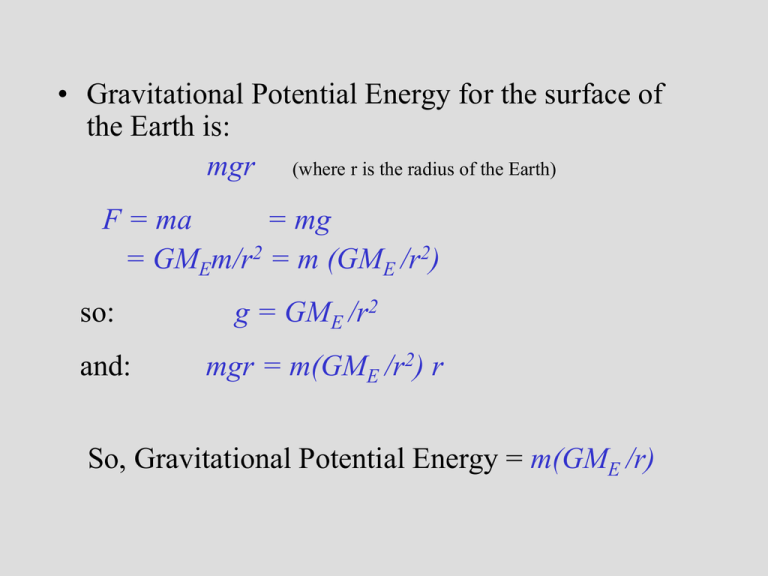
• Gravitational Potential Energy for the surface of the Earth is: mgr (where r is the radius of the Earth) F = ma = mg = GMEm/r2 = m (GME /r2) so: and: g = GME /r2 mgr = m(GME /r2) r So, Gravitational Potential Energy = m(GME /r) Escape Velocity set: ½ m v2 = GmME /r for a mass m to escape from the Earth (of mass ME) ½ v2 = GME /r vesc = 2GME /r Orbital Paths • Extending Kepler’s Law #1, Newton found that ellipses were not the only orbital paths. • possible orbital paths – ellipse (bound) – parabola (unbound) – hyperbola (unbound) Changing Orbits orbital energy = kinetic energy + gravitational potential energy conservation of energy implies: orbits can’t change spontaneously An object can’t crash into a planet unless its orbit takes it there. An orbit can only change if it gains/loses energy from another object, such as a gravitational encounter: If an object gains enough energy so that its new orbit is unbound, we say that it has reached escape velocity. What is light? Four Ways in Which Light can Interact with Matter emission absorption transmission reflection But, what is light? • In the 17th Century, Isaac Newton argued that light was composed of little particles while Christian Huygens suggested that light travels in the form of waves. • In the 19th Century, Thomas Young demonstrated that light bends slightly around corners and acts like interfering waves. Light A vibration in an electromagnetic field through which energy is transported. Light as a wave f=c Light as a particle E= a hf f photon Planck’s constant h = 6.6 x 10-34 J s Scottish physicist James Clerk Maxwell showed mathematically in the 1860s that light must be a combination of electric and magnetic fields. Anatomy of a Wave Light as a Wave • For a wave, its speed: v = f [distance/time] • But the speed of light is a constant c = 3 x 108 m/s • For light: f = c and f=c/ • The higher f is, the smaller is, and vice versa. • Our eyes recognize f (or ) as color! Light as a Particle • Light can also be treated as photons – packets of energy. • The energy carried by each photon depends on its frequency (color) E = hf = hc / (h = 6.6 x 10-34 J s) • Bluer light carries more energy per photon. Interaction of Light with Matter Hydrogen • Remember that each electron is only allowed to have certain energies in an atom. • Electrons can absorb light and gain energy or emit light when they lose energy. • It is easiest to think of light as a photon when discussing its interaction with matter. • Only photons whose energies (colors) match the “jump” in electron energy levels can be emitted or absorbed. Light as Information Bearer We can separate light into its different wavelengths (spectrum). By studying the spectrum of an object, we can learn its: 1 Composition 2 Temperature 3 Velocity











