Dilutions
advertisement

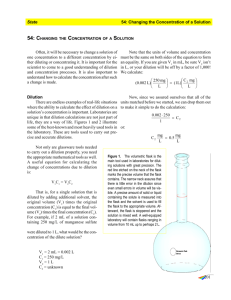
Microbiology lab (BIO 3126) 1 My coordinates • • • • • Instructor : John Basso Email : jbasso@uottawa.ca Office : Bioscience 102 Tel. : 613-562-5800 Ext. 6358 Web page: http://mysite.science.uottawa.ca/jbasso/home.htm 2 My Availability •Email : •All week before 5 •Tel. : •Mon - Fri : 9-5 3 Course Evaluation • Quiz – 2 bonus points for 100% on 4/8 quizzes • • • • • Assignments 2 Reports Midterm Exam 3MT presentations Practical Exam – In lab – Job Interview • Final Exam 20% 10% 25% 10% 5% 5% 25% Overview of web page • http://mysite.science.uottawa.ca/jbasso/home.htm 5 Microbiology Working in the microbiology lab At the beginning of the lab • Wash your hands as soon as you enter the lab – Helps to avoid contamination of the cultures with microorganisms from your natural flora Before starting –At the end • Disinfect your work area – Helps prevent contamination of cultures with microorganisms from the environment Before leaving the lab • Wash your hands before leaving the lab – Helps prevent contamination of the environment Working in Microbiology Sterile Technique The Material • The material used for the growth and handling of microorganisms must be sterile and maintained sterile – – – – – Growth media Tubes Petri dishes Inoculation loop Etc. • Use sterile technique for all transfers of microorganisms – Prevents contamination of your cultures – Prevents contamination of the environment – Prevents contamination of self • All bacteria are opportunistic Transfers Using Sterile Technique • Sterilize the inoculation loop with the Bunsen burner – The whole length of the wire must become Red • Do not deposit on the table! • Allow to cool down Boucle d’ensemencement Transfers Using Sterile Technique • Remove cap with your small finger of the inoculation loop hand – Do not put the cap on the table! Transfers Using Sterile Technique • Heat the mouth of the tube with the Bunsen burner – Keep the orientation of the tube as close as possible to the horizontal – Keep the opening of the cap downward Flame mouth of tube Transfers Using Sterile Technique • Use the sterile loop to remove inoculum – Liquid from broths – Solid from plates – Solid from slants Transfers Using Sterile Technique • Heat once again the mouth of the tube! – Keep the orientation of the tube as close as possible to the horizontal Flame mouth of tube Transfers Using Sterile Technique • Put the cap back on the tube of pure culture • Return the tube to the rack Transfers Using Sterile Technique • Repeat the same steps to inoculate a new tube – – – – – Remove cap Flame mouth of tube Inoculate Flame mouth of tube Close tube Inoculation Working with solutions Definitions • Solution – Mixture of 2 or more substances in a single phase – Solutions are composed of two constituents • Solute – Part that is being dissolved or diluted – Usually smaller amount • Solvent (OR Diluent) – Part of solution in which solute is dissolved – Usually greater volume Concentrations • Concentration = Quantity of solute Quantity of solution (Not solvent) • Four ways to express concentrations: – – – – Molar concentration (Molarity) Percentages Mass per volume Ratios Molarity • # of Moles of solute/Liter of solution – Mass of solute/MW of solute = Moles of solute – Moles of solute/vol. in L of solution = Molarity Percentages • Percentage concentrations can be expressed as either: – V/V – volume of solute/100 ml of solution – m/m – mass of solute/100g of solution – m/V – Mass of solute/100ml of solution • All represent fractions of 100 Percentages (Cont’d) • %V/V – Ex. 4.1L solute/55L solution =7.5% • Must have same units top and bottom! • %m/V – Ex. 16g solute/50mL solution =32% • Must have units of same order of magnitude top and bottom! • % m/m – Ex. 1.7g solute/35g solution =4.9% • Must have same units top and bottom! Mass per volume • A mass amount per a volume – Ex. 1kg/L – Know the difference between an amount and a concentration! • In the above example 1 litre contains 1kg (an amount) – What amount would be contained in 100ml? 100g 100% – What is the percentage of this solution? Ratios • A way to express the relationship between different constituents • Expressed according to the number of parts of each component – Ex. 24 ml of chloroforme + 25 ml of phenol + 1 ml isoamyl alcohol • Therefore 24 parts + 25 parts + 1 part • Ratio: 24:25:1 How many parts total? 50 Dilutions Reducing a Concentration A Fraction Dilutions • Dilution = making weaker solutions from stronger ones • Example: Making orange juice from frozen concentrate. You mix one can of frozen orange juice with three (3) cans of water. Dilutions (cont’d) • Dilutions are expressed as a fraction of the number of parts of solute over the total number of parts of the solution (parts of solute + parts of solvant) • In the orange juice example, the dilution would be expressed as 1/4, for one can of O.J. ( 1 part) for a TOTAL of four parts of solution (1 part juice + 3 parts water) Another example: • If you dilute 1 ml of serum with 9 ml of saline, the dilution would be written 1/10 or said “one in ten”, because you express the volume of the solution being diluted (1 ml of serum) per the TOTAL final volume of the dilution (10 ml total). Another example: • One (1) part of concentrated acid is diluted with 100 parts of water. The total solution volume is 101 parts (1 part acid + 100 parts water). The dilution is written as 1/101 or said “one in one hundred and one”. Dilutions (cont’d) • Dilutions are always a fraction expressing the relationship between ONE part of solute over a total number of parts of solution – Therefore the numerator of the fraction must be 1 – If more than one part of solute is diluted you must transform the fraction Example: • Two (2) parts of dye are diluted with eight (8) parts of solvent – The total number of parts of the solution is 10 parts (2 parts dye + 8 parts solvent) – The dilution is initially expresses as 2/10 – To transform the fraction in order to have a numerator of one, use an equation of ratios • The dilution is expressed as 1/5. Problem 1. Two parts of blood are diluted with five parts of saline – What is the dilution? 2/(2+5) = 2/7 =1/3.5 2. 10 ml of saline are added to 0.05 L of water – What is the dilution? 10/(10+50) = 10/60=1/6 Problem : More than one ingredient 1. One part of saline and three parts of sugar are added to 6 parts of water – What are the dilutions? Saline: 1/(1+3+6) = 1/10 Sugar: 3/(1+3+6) 3/10 = 1/3.3 2. How would you prepare 15mL of this solution? – Express each component being diluted over the same common denominator! Saline: 1/10 + Sugar 3/10 = 1.5/15 + 4.5/15 Serial Dilutions • Dilutions made from dilutions • Dilutions are multiplicative – Ex. – – – – A1: 1/10 A2: 1/4 A3: 0.5/1.5 = 1/3 The final dilution of the series = (A1 X A2 X A3) = 1/120 The Dilution Factor • Represents the inverse of the dilution • Expressed as the denominator of the fraction followed by “X” – EX. A dilution of 1/10 represents a dilution factor of 10X • The dilution factor allows one to determine the original concentration – Final conc. X the dilution factor = initial conc. Determining the required fraction (the dilution) Determine the reduction factor (The dilution factor) = What I have What I want Ex. You have a solution at 25 mg/ml and want to obtain a solution at 5mg/ml Therefore the reduction factor is: 25mg/ml 5mg/ml = 5 (Dilution factor) The fraction is equal to 1/the dilution factor = 1/5 (the dilution) Determining the amounts required • Ex. You want 55 ml of a solution which represents a dilution of 1/5 – Use a ratio equation: – 1/5 = x/55 = 11/55 • Therefore 11 ml of solute / (55 ml – 11 ml) of solvent • = 11 ml of solute / 44 ml of solvent Problem • Prepare 25mL of a 2mM solution from a stock of 0.1M – What is the dilution factor required? 50 – What is the dilution required? 1/50 – What volumes of solvent and solute are required? Solute 0.5ml Solvent 24.5ml Problem • How much of a 10M solution of HCl would you add to 18mL of water to obtain a 1M solution? – What is the dilution required? 1/10 – What volumes of solvent and solute are required? 1 part Solute / 10 parts of Solution 1 part Solute / 1 part Solute = 9 parts Solvent Therefore 9 parts solvent = 18mL or 1 part = 2mL