Protein Sorting & Transport: ER, Golgi, Lysosomes
advertisement

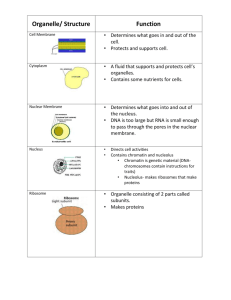
10 Protein Sorting and Transport Chapt. 10: Protein Sorting, Transport: Endoplasmic Reticulum, Golgi, Lysosomes Student learning outcomes: organelle functions • Explain sorting of proteins: free vs. bound ribosomes • Identify signals on proteins that identify destinations: Carbohydrates, amino acid sequences, lipids • Describe complexes that read signals, deliver cargo: SRP particles, Signal peptidases, chaperones, SNAREs • State steps taken for proteins destined for secretion, for a lysosome; state compartments traversed (compare nucleus); connected by vesicular transport Fig 10.1 The endoplasmic reticulum (ER) 1. Endoplasmic reticulum (ER); network of membrane-enclosed tubules and sacs (cisternae); extends from nuclear membrane through cytoplasm. • • • • Membrane is continuous; largest organelle of most cells. Rough ER is covered by ribosomes. Transitional ER is where vesicles exit to Golgi apparatus. Smooth ER no ribosomes, lipid metabolism Fig. 10.1 Fig 10.2 The secretory pathway ER and protein processing, secretion: • Pancreatic acinar cells secrete digestive enzymes • Newly synthesized proteins briefly labeled with radioisotopes, chased with nonradioactive • Proteins located by autoradiography. Fig. 10.2 Fig 10.3** Overview of protein sorting **Secretory pathway: • rough ER → Golgi → secretory vesicles → cell exterior Non-secreted proteins: • free ribosomes → nucleus, mitochondria, peroxisome **Fig. 10.3 The Endoplasmic Reticulum Ribosomes targeted to ER by signal sequence at NH2-terminus of polypeptide • Protein synthesis starts on free ribosomes Cotranslational translocation: • Proteins translocated into ER during synthesis on membrane-bound ribosomes. Figs. 8.22,27 Posttranslational translocation: • proteins are translocated into ER after translation completed on free ribosomes; Hsp70 Chaperone proteins Fig 10.5 Incorporation of secretory proteins into microsomes Evidence for signal sequences for ER: Secretory proteins translated from mRNAs on free ribosomes are larger than the secreted proteins. With microsomes, growing polypeptide chains incorporated into microsomes, signal sequences removed by proteolytic cleavage. Fig. 10.5 SDS-PAG analysis of proteins synthesized in vitro vs. secreted (s) Fig 10.6 The signal sequence of growth hormone Signal sequences (about 20 amino acids): • stretch of hydrophobic residues • usually located at NH2-terminus of polypeptide chain. Fig. 10.6 Signal sequence of growth hormone Fig10.8 Cotranslational targeting of secretory proteins to ER Cotranslational translocation: • Signal sequences are bound by signal recognition particle (SRP). • SRPs have 6 polypeptides, small cytoplasmic RNA (SRP RNA). SRP binds ribosome and signal sequence, binds SRP receptor on ER to make RER • Ribosome binds translocon; • Signal sequence inserts into translocon • Signal peptidase releases polypeptide in ER lumen Fig. 10.7,8 cotranslational Fig 10.9 Posttranslational translocation of proteins into ER Posttranslation translocation (more common in yeast): • Proteins synthesized on free ribosomes • Signal sequences recognized by receptors on translocon (not need SRP) • Hsp70 chaperones keep polypeptide chains unfolded so can enter translocon • Hsp70 chaperone in ER (BiP) acts as ratchet to pull polypeptide chain through Fig. 10.9 Posttranslational translocation The Endoplasmic Reticulum Transmembrane (integral) proteins: • Proteins destined for incorporation into membranes initially insert into ER membrane, not release into lumen. • Transported along secretory pathway as membrane components rather than soluble proteins • Membrane-spanning regions of integral membrane proteins usually α helical regions with ~20-25 hydrophobic amino acids. • Orientations vary — N or C terminus on cytosolic side • Some multiple membrane -spanning regions. Fig. 10.10 Fig 10.11 Topology of the secretory pathway ** Topology: Lumens of ER and Golgi are topologically equivalent to exterior of cell. Domains of plasma membrane proteins exposed on cell surface = regions of polypeptide translocated into ER lumen. Fig. 10.11 Fig 10.12 Insertion of membrane protein with cleavable signal sequence, single stop-transfer sequence Simplest method for Integral protein insertion: • Signal sequence cleaved by signal peptidase during translocation, leaves NH2-terminus in ER lumen. • Translocation halts at stop-transfer sequence; protein exits laterally and anchors in ER membrane. Fig. 10.12 Fig 10.13 Insertion of membrane proteins with internal noncleavable signal sequences Proteins also anchor in ER membrane by internal signal sequences not cleaved by signal peptidase. • These sequences are transmembrane α helices • Exit translocon, anchor proteins in ER membrane, in either orientation. Fig. 10.13 Fig 10.14 Insertion of protein that spans membrane multiple times Proteins that span the membrane multiple times are inserted by alternating series of internal signal sequences, transmembrane stop-transfer sequences. Fig. 10.14 Fig 10.15 Protein folding in the ER • Protein folding and processing occur either during translocation across ER or within ER lumen. • Lumenal ER proteins assist folding and assembly of translocated polypeptides • Hsp70 chaperone BiP binds to unfolded polypeptide chain as it crosses membrane, helps fold and assemble complexes • Disulfide (S—S) bond formation facilitated by PDI (protein disulfide isomerase). Fig. 10.15 The Endoplasmic Reticulum Glycosylation: • (N-linked glycosylation) occurs on specific asparagine (Asn) residues as protein translocates into ER. • Oligosaccharide is synthesized on lipid (dolichol) carrier. • Glycosylation prevents protein aggregation in ER, provides signals for sorting. Fig. 10.16 Fig 10.17 Addition of GPI anchors Glycosylphosphatidylinositol (GPI) anchors • Glycolipids attach some proteins to plasma membrane GPI anchors assemble in ER membrane, add to C-terminal Asn • GPI-anchored proteins are transported as membrane components via secretory path. • Topology within ER dictates they are exposed outside of cell. Fig. 8.36 GPI anchor Thy-1 Fig. 10.17 Fig 10.18 Glycoprotein folding by calreticulin Chaperones and sensors in ER identify misfolded proteins, divert them to degradation pathway. • Ex. chaperone calreticulin assists glycoproteins folding • Protein folding sensor passes correctly folded glycoproteins on to transitional ER. Fig. 10.18 The Endoplasmic Reticulum Unfolded protein response (UPR) BiP plays role as sensor of general state of protein folding. • If excess of unfolded proteins accumulates, BiP initiates UPR: • Includes general inhibition of protein synthesis, increased expression of chaperones, increase in degradation of mRNAs. Fig. 10.19 2. Smooth Endoplasmic Reticulum 2. Lipid synthesis occurs on smooth ER • Eukaryotic membranes 3 types of lipids: phospholipids, glycolipids, and cholesterol. • Phospholipids are synthesized on cytosolic side of ER from water-soluble precursors (glycerol, fatty acids) (Fig. 10.20). Fig. 10.20* The Endoplasmic Reticulum *Flippase moves phospholipids across membrane: • Synthesis of new phospholipids on cytosolic side keeps hydrophobic fatty acid chains buried in membrane. • Transfer to other half requires passage of polar head groups through membrane, facilitated by membrane flippases • Ensures even growth of both sides of phospholipid bilayer. Fig. 10.21 The Endoplasmic Reticulum Smooth ER: synthesis of cholesterol, ceramide. • Steroid hormones are synthesized from cholesterol in the ER • Ceramide is converted to glycolipids or sphingomyelin in the Golgi apparatus. • Smooth ER is abundant in cells with active lipid metabolism. • Smooth ER in liver has enzymes to detoxify lipid-soluble compounds Fig. 10.22 The Endoplasmic Reticulum – Vesicles ** Vesicles export proteins and phospholipid molecules from ER, bud from transitional ER, move through ERGolgi intermediate compartment, and then to Golgi. • Proteins in lumen of one organelle bud into transport vesicles, release to lumen of recipient organelle by vesicle fusion. • Membrane proteins and lipids are transported in similar way; topological orientation is maintained. Fig. 10.23* The Endoplasmic Reticulum ER Export: most proteins entering transitional ER are marked by sequences that signal export from ER. • Transmembrane proteins often di-acidic or di-hydrophobic amino acid signals in cytosolic domains. • GPI-anchored proteins are marked for export by GPI anchors. Fig. 10.24 The Endoplasmic Reticulum Return to the ER: • Some proteins must stay in ER (BiP, signal peptidase, etc). • Target sequence (KDEL or KKXX) at carboxy terminus directs retrieval from ERGIC or Golgi complex via recycling path. Fig. 10.25 The Golgi Apparatus 3. the Golgi apparatus, or Golgi complex: • Proteins from ER are processed and sorted for transport: to endosomes, lysosomes, plasma membrane, or secretion. • Most glycolipids and sphingomyelin are made in Golgi, • The Golgi is composed of flattened membrane-enclosed sacs (cisternae) and associated vesicles: • cis Golgi network receives molecules from ERGIC • medial and trans Golgi stacks most modifications here • trans Golgi network sorting and distribution Fig. 10.26 The Golgi Apparatus Mechanism of protein movement is not certain; they may be carried in cisternae, which gradually mature, move through Golgi in cis to trans direction. Transport vesicles return Golgi resident proteins for reuse. Fig. 10.27 The Golgi Apparatus Glycoproteins are processed in the Golgi: • Glycosyltransferases add different sugar residues; glycosidases remove sugar residues • modification of N-linked oligosaccharides added in ER. • Mannose residues are removed; N-acetylglucosamine, galactose, and sialic acid residues are added. • Proteins emerge with different N-linked oligosaccharides Fig. 10.28 Golgi modifications The Golgi Apparatus *Lysosomal proteins: N-linked oligosaccharides modified by mannose phosphorylation. • Enzyme recognizes structural signal (shape) on folded lysosomal proteins: signal patch, not a linear aa sequence. • Lysosomal protein binds mannose-6-phosphate receptor in trans Golgi, which transports it to endosomes and lysosomes. Fig. 10.29 The Golgi Apparatus Glycolipids • synthesized from ceramide by addition of carbohydrates. Sphingomyelin (nonglycerol phospholipid in membranes) • made by transfer of phosphorylcholine group from phosphatidylcholine to ceramide. Fig. 10.30 Made in smooth ER Fig 10.31 Transport from the Golgi apparatus trans Golgi sorts, packages molecules in transport vesicles Proteins staying in the Golgi bind the membrane; signals preventing packaging and transport. Intracellular destinations: late endosome, lysosome, vacuole (yeast, plants lack lysosome) Transport to cell surface : – Direct transport – Recycling endosomes – Regulated secretory paths (vesicles) Fig. 10.31 The Golgi Apparatus Regulated secretion pathways: • release of hormones from endocrine cells • neurotransmitters from nerve cells. • digestive enzymes from pancreatic cells • These proteins have patch signals recognized by cargo receptors. • released in secretory vesicles: contents stored until signals direct fusion with plasma membrane. Fig. 10.31 Fig 10.32 Transport to the plasma membrane of polarized cells Polarized epithelial cells have plasma membrane divided into apical domain, and basolateral domain: each has specific proteins. Proteins leaving trans Golgi selectively packaged, transported to correct domain. Fig. 10.32 The Mechanism of Vesicular Transport Selectivity of vesicular transport is key to functional organization of a cell. • Vesicles must recognize and fuse only with appropriate target membrane. • Understanding mechanisms that control vesicular transport is major area of research in cell biology. • Includes secretion and uptake of molecules (Ch. 13) Experimental approaches to understanding transport include: Isolation of yeast mutants defective in transport and sorting (sec mutants) Reconstitution of vesicular transport in cell-free systems Biochemical analysis of synaptic vesicles (neurons) Tracing path of specific GFP fusion proteins through secretory network Proteomic analysis of secretory compartments The Mechanism of Vesicular Transport Cargo selection, coat proteins, vesicle budding Transport vesicles with secretory proteins have cytosolic coat proteins. Coats assemble as vesicle buds; are removed in cytosol before vesicle reaches its target. Vesicles fuse with target membrane, empty cargo, and insert their membrane proteins into target membrane. Fig. 10.34 The Mechanism of Vesicular Transport Three families of vesicle coat proteins COPII-coated vesicles carry secretory proteins from ER to ERGIC to Golgi apparatus COPI-coated vesicles bud from ERGIC or Golgi, carry cargo backwards, return proteins to earlier compartments. *Clathrin-coated vesicles transport in both directions between trans Golgi network, endosomes, lysosomes, and plasma membrane. Fig. 10.35 Fig 10.37 Incorporation of lysosomal proteins into clathrin-coated vesicles Clathrin-coated vesicles require • • • • clathrin, GTP-binding protein ARF1, and adaptor proteins Clathrin assembles into basketlike lattice that distorts membrane, starts bud Proteins targeted for lysosomes have Mannose-6-PO4, binds Mannose-6-PO4 receptors in trans Golgi membrane Mannose-6-PO4 receptors span the Golgi membrane, binding sites for cytosolic adaptor proteins, which bind clathrin Clathrin-coated vesicles have many destinations; different adaptors Fig. 10.37 The Mechanism of Vesicular Transport SNARE hypothesis: Vesicle fusion occurs by interactions between pairs of transmembrane proteins, called SNAREs, on vesicle and target membranes (v-SNAREs, t-SNAREs). SNARE-SNARE pairing provides energy to bring two bilayers close enough to destabilize, permit fusion Rab family of small GTP-binding proteins plays key role in vesicle budding and fusion: • Mark different organelles and transport vesicles, so need to be localized on correct membrane for specificity Rab proteins have hydrocarbon prenyl tail to insert in membrane Fig 10.39 Vesicle fusion SNARE hypothesis Rab/GTP on transport vesicle interacts with effector proteins and v-SNAREs to assemble pre-fusion complex • Different Rab protein on target membrane organizes other effector proteins and t-SNAREs. • Effector proteins link membranes by proteinprotein interactions (tethering). • After fusion, protein complex (NSF/SNAP complex) disassembles SNAREs for reuse Fig. 10.39 SNAREs Fig 10.41 Electron micrograph of lysosomes, mitochondria in mammalian cell 4. Lysosomes • Membrane-enclosed organelles with ~ 50 acid hydrolases • • Enzymes break down diverse macromolecules Digestive system of the cell, recycles organelles also. Fig. 10.41 lysosomes (→) and mitochondria Lysosomes and disease Lysosomal storage diseases: • Mutations in genes encoding lysosomal enzymes: • Undegraded material accumulates, • Impairs macrophages, neural cells. • Ex. Gaucher disease is glucocerebrosidase defect • Treat with enzyme replacement therapy Lysosomes • Most lysosomal enzymes are acid hydrolases: active at pH 5 in lysosomes, not cytoplasm (pH 7.2). • Acid activity protects against uncontrolled digestion of cell contents; • If lysosome membrane breaks, acid hydrolases are inactive in cytosol. • To maintain acidic pH, a proton pump in lysosomal membrane actively transports protons Fig. 10.42 Fig 10.43 Endocytosis and lysosome formation Lysosomes digest material taken up from outside by endocytosis. (Chapt. 13) Endosomes are intersection between secretory and endocytic pathway Endocytosis and lysosome formation: Early endosomes Separate molecules for recycling (e.g. receptors) from those for degradation Molecules to be recycled go to recycling endosomes and back to plasma membrane. Late endosomes receive lysosomal enzymes from trans Golgi network, can fuse or mature into lysosomes Fig. 10.43 Lysosomes • Phagocytosis: • Specialized cells such as macrophages take up, degrade large particles (bacteria, cell debris, aged cells. • Particles in phagocytic vacuoles (phagosomes), fuse with lysosomes to become phagolysosomes. • Autophagy: • turnover of cell contents old organelles Fig. 10.44 Review Review questions: 1. Describe experimental evidence for secretory path: ER -> Golgi -> secretory vesicle -> secreted protein 3. Compare/ contrast co-translational with posttranslational translocation of polypeptides into ER 5. Why are carbohydrate groups of glycoproteins always exposed on the surface of the cell? Review 6. What would be effect of mutating KDEL sequence of resident ER protein like BiP? How would this differ from mutating the KDEL receptor protein? 7. How is a lysosomal protein targeted to lysosome? What about adding a lysosome-targeting signal patch on protein that is normally cytosolic? 9. What processes result in glycolipids and sphingomyelin being found in outer – but not innerhalf of plasma membrane bilayer?