Word
advertisement

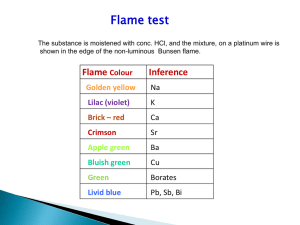
CHM202 General Chemistry and Laboratory II Laboratory 8 Inorganic Qualitative Analysis Name Section Group Unknown # Results 1. Tests for Silver (I) Ion (Group I) Start with 30 drops of sample [1.2] 2 Drops 6M HCl [1.2] No Precipitate Precipitate Known - Contact Instructor Unknown - Go to 2.1 (test for Cu2+) [1.3] Centrifuge then add 1 drop 6M HCl [1.3] Centrifuge and separate solid and solution [1.4] Solid Add 6 drops 6M NH3, mix [1.5] Ag+ ID - White ppt? Known Unknown Additional Precipitate? Repeat 1.3 until no more ppt is observed [1.3] Some solid remains Add 3 drops NH3 [1.5] Solution Save for 2.1 [1.4] Centrifuge and Solution Ag+ Confirm. separate solid and solution [1.6] Add 6M HNO3 Precipitate should form [1.6] Known Unknown The known solution tested positive for silver (I) ion YES NO The unknown solution tested positive for silver (I) ion YES NO 2. Tests for Copper (II) Ion (Group II) Start with solutions from 1.3 or 1.4 Add 6M NH3 until basic [2.1] Estimate volume + .6mL; add 1 drop HCl per mL of soln Test pH< 2 [2.2] Add 12 drops TA Heat 8-10 min [2.3] Cu+2 ID - Black ppt? Known No Precipitate Known - Contact Instructor Unknown - Go to 3.1 (test for Zn2+) [2.4] Solid Add 20 drops 6M HNO3 Heat 2-3 min Centrifuge and separate [2.7] Unknown Precipitate Centrifuge separate solid and solution [2.4] Solution Add 3 drops TA Heat 5 minutes Centrifuge if ppt Combine solid with solid from 2.4 [2.5] Combined Solids Add 20 drops of Distilled Water, Mix Centrifuge/separate [2.6] Solution Save for 3.1 [2.5] Solution Add 6M NH3 until basic, add 6M HOAc until acidic [2.8] Est. vol. + 0.6 mL drops of HCl = pH <2? (If not add more HCl) Solution Discard to waste [2.6] Cu2+ Confirm. Add 10 drops of K4Fe(CN)6 Red-brown ppt confirms Cu2+ Heat content 3 min if no ppt. [2.9] Known Unknown The known solution tested positive for copper (II) ion YES NO The unknown solution tested positive for copper (II) ion YES NO 3. Test for Zinc Ion (Group III) Start with solutions from 2.4 or 2.5 Add 10 drops 6M HCl Add 6M NH3 until basic [3.1] Add 12 drops Thioacetamide Heat 8-10 min [3.3] No Precipitate Known - Contact Instructor Unknown - Go to 4.1 (test for Ca2+) [3.4] Zn2+ ID - White ppt? Known Unknown Precipitate Centrifuge separate solid and solution [3.4] Solution Add 3 drops TA Heat 5 minutes Centrifuge if ppt Combine solid with solid from 3.4 [3.5] Combined Solids Add 20 drops of Water + 2 drops of 6M NH3, Mix, Centrifuge and Separate [3.6] Solution Save for 4.1 [3.5] Solid Add 20 drops 6M HNO3 Heat 2-3 min Centrifuge and separate [3.7] Add 5 drops 6M NH3 pH must be > 9 If not add more NH3 [3.2] Add 6M NH3 until basic, add 6M HOAc until acidic [3.8] Solution Discard to waste [3.6] Zn2+ Confirm. Add 10 drops of K4Fe(CN)6 White ppt confirms Zn2+ [3.9] Heat 3 min if no ppt. Known Unknown The known solution tested positive for zinc (II) ion YES NO The unknown solution tested positive for zinc (II) ion YES NO 4. Tests for Calcium Ion (Group IV) Start with solutions from 3.4 or 3.5 [4.1] Add 10 drops 3M (NH4)2CO3 [4.1] Ca2+ ID - White ppt? Known Unknown No Precipitate Known - Contact Instructor Unknown - Test Complete (neg. for Ca2+) [4.1] Precipitate Centrifuge Separate solid Add 10 drops water, mix, Centrifuge Separate solid [4.2] Solution Add 6M NH3 until basic [4.4] Ca2+ Confirm. Add 10 drops K4Fe(CN)6 or Add 10 drops K2C2O4 White ppt? If no, heat [4.4] White ppt? [4.5] If a white precipitate forms from either confirmation test then calcium ion was present in the solution. Known Solid Add 5 drops 6M HOAc or more if not dissolved [4.3] Unknown The known solution tested positive for calcium ion YES NO The unknown solution tested positive for calcium ion YES NO Questions 1. Write the Ksp expression for each of the precipitation reactions used to identify each group and calculate the concentration of the anion required to precipitate a 0.1 M cation solution for each of these. 2. Predict if the following conditions would result in the formation of a precipitate: a) 0.0025 M Fe3+ at pH 6.00 b) 0.0025 M Ba2+ and a 1.25 x 10-4 M solution of sodium sulfate c) 0.05 M Ca2+ and a 1.25 x 10-4 M solution of cesium phosphate 3. Determine the equilibrium concentration of the following: a) The cobalt ion concentration of a saturated solution of CoS b) The pH of a saturated Zn(OH)2 solution c) The lead (II) ion concentration of a saturated solution of PbCl 2.