Cabbage Lab Part 1: Standardization Procedure: Add a squirt of
advertisement

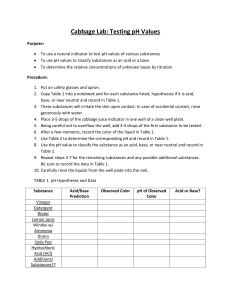
Cabbage Lab Part 1: Standardization Procedure: 1. Add a squirt of each buffer into a test tube 2. Add just enough indicator (cabbage juice) to see a color change 3. Record the color change. 4. Save your test tubes to serve as color references for Part 2 Data: Use a very basic color vocabulary so that someone else looking at your data would understand it. pH Color Observed 0 2 3 4 5 6 7 8 9 10 11 12 14 Cabbage Lab: Crude Titration Procedure: 1. Put 15 drops 1M NaOH into a test tube 2. Add 5-10 drops of cabbage indicator 3. Add 30 drops HCl drop by drop. After each drop, record the pH. Data: Drops Approx. pH 0 17 1 18 2 19 3 20 4 21 5 22 6 23 7 24 8 25 9 26 10 27 11 28 12 29 13 30 14 15 16 Conclusion: 1. Plot pH vs. # of drops. Fit your plot with a smooth curve and clearly mark the neutral point on the curve. 2. Which is stronger based on molarity- the acid or the base? Explain.