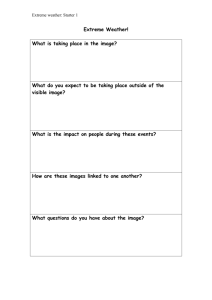
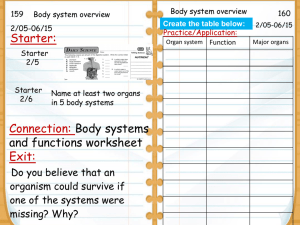
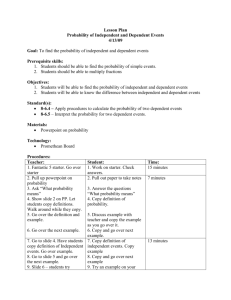
Apr20 - Trimble County Schools
advertisement

April 20th, 2015 Ms. Angela Pacheco Trimble County High School Science Department Astronomy • • • • Starter: get out SW/APOD sheet SW/APOD Go over Mars exam Continue Jupiter unit 2nd Period Chemistry I • Starter: get out your calculator and writing utensil • Practice problems • 10-2 Apply wkst • Summative exam: tomorrow • 2nd chance for learning check #3: Tues or Thur in Mrs. Green’s room Chemistry I Practice Problems 1. 2. 3. 4. 5. 6. 7. 8. What is the formula mass of CCl4? Convert 67.3 grams of H3PO4 to moles. What is the atomic mass of Phosphorus? How many molecules of Ca(NO3)2 are in 23.4 grams? Convert 57.3 moles of nitrogen gas into Liters at STP. Convert 267.3 grams of H2SO4 to molecules. What is the molar mass of pentane (C5H12)? A storage tank of helium has a volume of 500. L contains how many moles of He at STP? 9. A sample of iron oxide has a mass of 3.192 g. It is analyzed and found to contain 2.232 g of iron and 0.96 g of oxygen. Find the percentage composition of this compound. 10. Find the molecular formula of a compound that contains 40% carbon, 6.7% hydrogen and 53.3% oxygen. The molar mass of the compound is 180 g/mol. Chemistry Groups 1 1 2 2 3 3 Diane Zhoe Autumn Brantley Hannah Joseph Lily Johnny Amber Rhiannon David Chandler Sarah Aaron Kat Easton Calvin Brooke Kalani Anissa Angela 4 4 Morgan 5 5 6 6 7 7 Conceptual Physics • Starter: get out SMQ sheet • SMQ • Reading and notes: – Pgs. 191 Eclipses – Pgs. 187-192 Ocean Tides • Learning check #1: Wednesday • End of class: 2 minute reflection and column • Learning check #3 2nd chance: Tues if you signed up Advisory • Starter: pass out hardback books • Reading: Seek First to Understand, Then to Be Understood, pg. 176 • Habit 6: Synergize, pg. 182 AP Chemistry Prep • Starter: get out your calculator, formula sheet and periodic table • Sample free response question • Score question with rubric Astronomy • • • • Starter: get out SW/APOD sheet SW/APOD Go over Mars exam Continue Jupiter unit Advanced Physics • • • • Starter: get out SMQ sheet SMQ Reading, pg. 167 - Torque Group problems, – Set 2: pg. 170 (10-12, 49-51) – Set 3: pg. 756 (17, 16, 14, 15) • Learning check: Tuesday Advanced Physics 1 2 3 4 5 6 7 Morgan K Tailor Amber Madelyn Brennan Danielle Morgan C Brady Bret Logan Alex Nick Emily T Courtney Mila Tanya Collin Sidney Dalton Zach Raven Megan W Michael Katie Emily A David Megan T Eli AP Chemistry Agenda • Starter: • Equation writing practice • Sample free response problem • Sample multiple choice questions AP Chem Balancing Practice • A solution of nickel (II) chloride is added to a solution of sodium hydroxide, forming a precipitate. – Write the balanced net ionic equation. – If equal volumes of 1.0 M nickel (II) chloride and 1.0 M sodium hydroxide are used, what ion is present in the solution in the highest concentration after the precipitate forms?