lecture6
advertisement

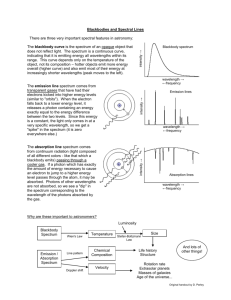
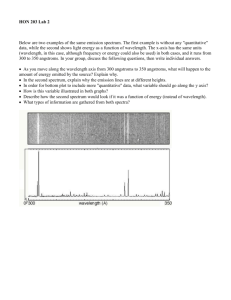
Astronomy Picture of the Day Light Radiation and Spectra Chapter 5 What is Light? ● ● Newton – Prism shows white light contains all colors – Light made of particles (photons) Maxwell – Theory of electricity and magnetism – Light is electromagnetic waves ● ● ● Produced by wiggling electrons Radiation = production of light Quantum Mechanics – Light is both: particle and wave Waves ● Wavelength ( l ) – ● Distance between crests (or troughs) Frequency ( f ) – How often it repeats (wiggles up and down) ● Measured in Hertz (Hz) – number of times per sec Waves ● Speed c = 3 x 108 m/s c = lf ● Wavelength inversely related to frequency l=c/f – high frequency = short wavelength – low frequency = long wavelength Particles as Waves ● “Wave Packet” – particle/photon = localized wave Properties of Light Color ● Depends on frequency – ● blue = high frequency = short wavelength ● red = low frequency = long wavelength Carries energy (heat) ● Photon energy – E=hf ● high frequency = high energy = blue ● low frequency = low energy = red h = Planck’s constant Red light has ____ than blue light. A. larger frequency, energy, and wavelength B. smaller frequency, energy, and wavelength C. larger frequency and energy, but smaller wavelength D. smaller frequency and energy, but larger wavelength Which of the following travels fastest? A. B. C. D. E. } radio waves infrared (heat) waves microwaves blue light waves none of the above All are types of light! All types of light travel at the same speed the “speed of light”, c CPS Question ● ● ● ● ● The color of visible light is determined by its ____. A) brightness B) amplitude C) speed D) wavelength CPS Question ● ● ● ● If the wavelength of light increases, the frequency must ____. A) increase also B) decrease C) remain unchanged CPS Question ● ● ● ● ● The bending of light that occurs when moving between media of different densities is called ___. A) reflection B) refraction C) diffraction D) distortion Propagation of Light ● Photons travel in straight lines – energy spread over larger area at larger distances – produces 1/r2 decrease in brightness ● Double distance - brightness decreases by 4 If a 100-watt light bulb is placed 10 feet away from you, and an identical 100-watt light bulb is placed 100 feet away from you, which will appear brighter? A. The closer one B. The farther one C. They will appear the same brightness How much fainter will the far one appear compared to the close one? A. B. C. D. Twice as faint 10 times fainter 100 times fainter 1000 times fainter ~ 1/r2 The Electromagnetic Spectrum 1 nm = 10 -9 m , 1 Angstrom = 10 -10 m c = lf Electromagnetic Spectrum ● Visible light: – ● red, orange, yellow, green, blue, indigo, violet (ROYGBIV) Invisible Light: – Ultraviolet = bluer than blue – Infrared = redder than red – Other wavelengths: ● Short: X-rays, gamma-rays ● Long: microwave, radio Thermal Radiation ● All objects radiate (thermal radiation) – Objects made of atoms – Atoms (and their electrons) vibrate ● – Bigger objects produce more light – Higher temperature = stronger vibration ● ● Wiggling electrons radiate, producing light Hotter objects emit more light Perfect absorber is black – – Absorbed light (energy) heats object Temperature increases until emitted energy = absorbed energy – ● Emitted radiation called Blackbody Radiation Thermal radiation emitted by most objects similar What does the spectrum of an astronomical object's radiation look like? Many objects (e.g. stars) have roughly a "Black-body" spectrum: Brightness Frequency also known as the Planck spectrum or Planck curve. Blackbody Radiation Laws ● Luminosity, L L = energy emitted per second ● Luminosity for a spherical object (a star) L = 4pR2 s T4 Stefan-Boltzmann Law R = radius (size) of star; T = temperature – double size, luminosity increases by 2x2 = 4 – double temperature; luminosity increases by 2x2x2x2 = 16 Spectroscopy and Atoms How do you make a spectrum? Refraction of light When you bend light, bending angle depends on wavelength, or color. Questions ● ● How is temperature related to the amount of energy radiated? How is temperature related to the color of the object? (Blackbody Demo) The wavelength of peak emission tells us the temperature of the object! "cold" dust "cool" star Sun "hot" stars frequency increases, wavelength decreases Blackbody Radiation Blackbody Radiation Laws ● Color – Wavelength where most light emitted lmax = 3 x 106 / T T in Kelvin; lmax in nanometers (1 nm=10-9m) – ● Cool stars are red ● Hot stars are blue Color indicates temperature! As T As T , Wavelength , Wavelength , Color = redder , Color = bluer Wien’s Law The graph above shows blackbody spectra for three different stars. Which of the stars is at the highest temperature? Because peak energy emission A. Star A occurs at shortest wavelength B. Star B Doppler Shift ● Originally discovered using sound waves ● Moving object – ● emits light with slightly different color Frequency (pitch) of approaching object is higher – Blueshift ● ● Wavelength shorter (shifted blueward) Frequency (pitch) of receeding object is lower – Redshift ● Wavelength longer (shifted redward) video Video Doppler Shift Redshift Blueshift Spectroscopy ● Prism separates light into different colors – Continuous spectrum ● contains all colors ● Example: blackbody spectrum Spectroscopy Absorption Line Spectrum – Some colors are missing (discrete lines) Solar Spectrum N.A.Sharp, NOAO/NSO/Kitt Peak FTS/AURA/NSF Spectroscopy – Emission Line spectrum ● Only certain colors are present (discrete lines) ● Spectrum for each element unique (like fingerprints) Pattern of lines is a fingerprint of the element For a given element, emission and absorption lines occur at the same wavelengths. Helium discovered in Sun’s spectrum before being found on Earth! Sodium emission and absorption spectra Spectrum of the Sun • Absorption spectrum • What causes emission/absorption of light at specific wavelengths? Interactive Video 1, 2, 3 Types of Spectra 1. "Continuous" spectrum 2. "Emission" spectrum 3. "Absorption” Spectrum video The Particle Nature of Light Light interacts with matter as individual packets of energy, called photons. c photon energy is proportional to frequency: 1 E f (or E l example: ultraviolet photons are more harmful than visible photons. Model Atom ● Nucleus – – contains protons and neutrons number of protons = element hydrogen (1 proton = hydrogen, 2 protons = helium, etc.) – number of neutrons about same as protons ● Isotope = different number of neutrons helium Isotopes of hydrogen Model Atom ● Electrons orbit nucleus – Number of electrons = number of protons ● – Ionization = removing electrons Only certain orbits are allowed hydrogen helium The Nature of Atoms The Bohr model of the Hydrogen atom: electron _ _ + + proton "ground state" an "excited state" (Fair Analogy) Atomic Absorption ● Atom absorbs photon energy – electron “jumps” to higher energy orbit – only certain discrete orbits are allowed ● Atom can absorb only discrete colors (energies) When an atom absorbs a photon, it moves to a higher energy state briefly When it jumps back to lower energy state, it emits photon(s) in a random direction, conserving the total energy of the system! Atomic Emission ● Electron “jumps” to a lower energy orbit – Atom emits photon – can emit only discrete colors ● same colors (wavelengths/energies) as absorption Atomic Energy Levels ● Energy Levels – Different for each element ● each element has unique set of absorption/emission lines Other elements Helium neutron Carbon proton Each element has its own allowed energy levels yielding a unique spectral fingerprint. Kirchoff’s Laws ● Continuous spectrum – ● Emission line spectrum – ● Produced by hot solid (or dense gas) Produced by hot, low density gas Absorption line spectrum – Produced when continuous source is viewed through cooler low density gas Kirchoff’s Laws ● Absorption lines same wavelengths as emission lines – Gas can only absorb and emit at certain discrete frequencies/wavelengths/energies video If you analyze the light from a low density object (such as a cloud of interstellar gas), which type of spectrum do you see? A. dark line absorption spectrum B. bright line emission spectrum C. continuous spectrum Imagine that you observe the Sun while in your space ship far above Earth’s atmosphere. Which of the following spectra would you observe by analyzing the sunlight? A. dark line absorption spectrum B. bright line emission spectrum C. continuous spectrum CPS Question Which ONE of these is constant for all forms of EM radiation in a vacuum? A) amplitude B) wavelength C) frequency D) speed E) energy CPS Question Which ONE is NOT a property of a blackbody? A) It appears black, regardless of its temperature. B) It emits radiation in a continuum of wavelengths. C) Its spectrum peaks at a wavelength determined by its temperature. D) The total energy that it radiates increases rapidly with temperature. CPS Question The Sun's observed spectrum is _____. A) A continuum with no lines, like the rainbow. B) A continuum with bright emission lines. C) Only absorption lines on a black background. D) Nearly a continuum with some absorption lines. Ionization Hydrogen _ _ + + _ _ Helium + + + + _ _ "Ion" Absorbing a high energy photon and atomic collisions can both lead to ionization. Spectrum of the Sun • • • Complicated objects => many different elements Nearly continuous absorption spectrum What causes emission/absorption of light at certain wavelengths? Why emission lines? hot cloud of gas . . . . . . - Photon absorption/atomic collisions excite atoms - Electron drops back to lower level - Photons at specific frequencies emitted Why absorption lines? . . . . . . cloud of gas . . (Shockwave Demo) (Web Link) . . . Stellar Spectra Sun's 'atmosphere' specific wavelengths Fusion generates continuous spectrum Star absorbs Kirchhoff's Laws 1. Continuous spectrum 2. Emission spectrum 3. Absorption spectrum Question How does the pitch or tone of a sound wave change when the source of the sound is moving towards or away from you? What about when you are moving towards or away from the source? Does this effect occur for all types of waves or just for sound waves? Doppler Shifted Atomic Spectra • Why don’t we see the color of everyday objects change as they move? We've used spectra to find planets around other stars! (Ch. 4) • Star wobbling causes Doppler shift of its absorption lines. • Only gives information about velocity along line of sight!