Water
advertisement
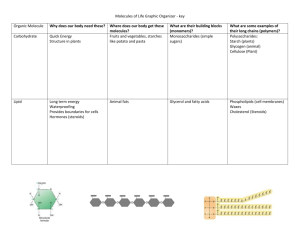
Water A. B. C. D. E. Structure of water Ionization of water Solvent properties of water Thermal properties of water Monomers and polymers A. Structure of Water A water molecule is composed of two hydrogen atoms covalently bonded to an oxygen atom The hydrogen atoms form an angle of about 110°, so the molecule is “bent” A. Structure of Water The oxygen nucleus exerts a greater “pull” on the electrons in the covalent bonds – Therefore, the oxygen atom has a partial negative charge – And the hydrogen atoms have partial positive charges A. Structure of Water Because opposite charges attract each other, water molecules are attracted to each other and to other charged molecules or ions (This is an example of a hydrogen bond.) A. Structure of Water The bent geometry of water and the attraction between water molecules gives rise to unique properties that are essential for its role in living organisms and the environment B. Ionization of Water Consider a glass of pure water: – In a tiny fraction of the water molecules (1 out of 10 million), one of the hydrogen nuclei is completely pulled off the molecule – This forms two ions: • A hydrogen ion (H+) • And a hydroxyl ion (OH–) – This is caused by the attraction of the water molecules for each other B. Ionization of Water Water molecules are continuously splitting into ions and rejoining to form water molecules B. Ionization of Water In chemically pure water, the number of H+ and OH– ions are the same Certain chemical substances, when dissolved in water, can change the amounts of H+ or OH– B. Ionization of Water Acid – A substance that increases the amount of H+ (and decreases the amount of OH–) Base (Alkaline) – A substance that increases the amount of OH– (and decreases the amount of H+) Neutral substance – A substance that does not change the amounts of H+ and OH– (so H+ remains equal to OH–) B. Ionization of Water Acidity and alkalinity are represented by a value called “pH” – – – – – pH = -log[H+] Acids: pH value is less than 7 Bases: pH value is greater than 7 Neutral substances: pH value is equal to 7 Each pH value represents a 10-fold change in the amount of H+ in the solution – So a substance with pH = 5 has a 10 times greater amount of H+ than a substance with pH = 6 C. Solvent Properties of Water Solution – A mixture of two (or more) different substances in which the particles of one substance are completely interspersed with the particles of the other substance(s) – Solvent: The substance that is present in the largest amount – Solute: The substance(s) that are present in smaller amounts C. Solvent Properties of Water Hydrophilic substances – Substances that can be dissolved in water – Water molecules are attracted to ions or to other molecules that have partial positive and negative charges – Examples of hydrophilic substances: • Sodium chloride (table salt): This substance consists of sodium ions and chloride ions • Sucrose (table sugar): This substance is a compound with many -OH groups in its structure, with many partial positive and negative charges C. Solvent Properties of Water Hydrophobic substances – Substances that cannot be dissolved in water – Water molecules have difficulty interacting with uncharged molecules. These substances tend to separate from water. – Example of a hydrophobic substance: • Cooking oil: The molecules of cooking oil have long chains of carbon atoms bonded to hydrogen. The atoms do not have the “bent” geometry of water, so there are no partial charges to attract the water. Therefore, oil and water don’t mix! C. Solvent Properties of Water Amphipathic substances – Substances in which part of the molecule is hydrophobic, and part of the molecule is hydrophilic – When amphipathic substances are mixed in water, its molecules form into clusters called “micelles” • with the hydrophilic part on the outside of the micelle in contact with water • and the hydrophobic part on the inside of the micelle, away from the water . C. Solvent Properties of Water Amphipathic substances (cont.) – Example of an amphipathic substance: • Soap: Soap molecules have an ionic group attached to one end, and an oily hydrocarbon chain attached to the other end. When soap is mixed with water, it forms micelles that trap oily dirt molecules. D. Thermal Properties of Water Molecules are in constant motion due to the heat energy (kinetic energy) they contain Phases of matter: – Solid • Limited movement of molecules; non-fluid – Liquid • Molecules can move freely around each other; fluid – Gas • Molecules have greatest freedom of movement; substance can expand to fill the available space D. Thermal Properties of Water Water has unusual thermal properties because of the attraction of water molecules for each other – Water has relatively high melting and boiling points – Water remains in a liquid state over a wide temperature range – Water has a high heat capacity: it can absorb a large amount of heat with a small change in temperature – The solid form of water (ice) is less dense than the liquid, so ice floats on water E. Monomers and Polymers Monomer – An organic molecule that serves as a “building block” to build larger organic molecules Polymer – An organic molecule composed of two or more monomer units linked together by covalent bonds E. Monomers and Polymers Condensation reaction – Polymers are often formed by the process of condensation – In this process, two hydrogen atoms and an oxygen atom are removed from two monomer units – And a covalent bond forms between the monomers E. Monomers and Polymers E. Monomers and Polymers Hydrolysis reaction – Polymers are often broken down by the process of hydrolysis – In this process, a water molecule is inserted between the monomer units of a polymer – To split the polymer into its monomer units E. Monomers and Polymers