Zumdahl's Chapter 15
advertisement

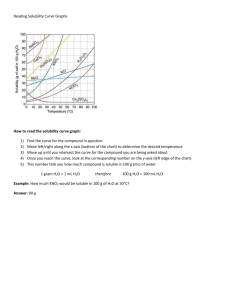
Zumdahl’s Chapter 15 Applications of Aqueous Equilibria Chapter Contents Acid-Base Equilibria Common Ion Effect Buffers Titration Curve Indicators Solubility Solubility Product Common Ion Effect pH and Solubility Complex Equilibria Complexes and Solubilities Acid-Base Titrations Le Châtlier: restoration of equilibrium replaces species lost. QK E.g., H2O is a weaker electrolyte than virtually any other weak acid, so … Titrating weak acid with strong base binds proton in water, removing product! such titrations are quantitative. Common Ion in Acid-Base Le Châtlier: restoration of equilibrium consumes addends. QK Addition of an ion already in equilibrium (Common Ion Effect) restores K by consuming the common ion. NH3 + H2O NH4+ + OH– Kb=1.810–5 0.1 M NH3 [OH–] [1.810–50.1]½ = 410–3 Make it 0.1 M NH4+ and [OH–] 1.810–5 ! Buffer Solutions Kb = [BH+][OH–]/[B] Ka = [H+][A–]/[HA] If [B]=[BH+], then [OH–] = Kb If [HA]=[A–], then [H+] = Ka Furthermore, in either case, excess H+ or OH– finds abundance of its reactant! Associated robust pH, a buffer hallmark. Buffer Calculation 0.1 M ea. [NH4+] & [NH3]; pOH = 4.74 100 mL of this buffer contains 10 mmol of each of those species. React fully 5 mmol OH– (in same 100 mL) Kb = (0.1–0.05+x) (0+x) / (0.1+0.05–x) x = [OH–]new (3 Kb)½ or pOHnew = 4.27 5% rule OK due to starting point of full reaction! pOH = 0.47 trivial given even a 50% addend! Titration Curves While [HA]/[A–] or [B]/[BH+] not near zero, buffering makes pH near pK pH changes slowly near ½ completion. Near endpoint, those ratios vanish making [H+] very sensitive to titrant. pH changes very rapidly near endpoint! Titration: Weak Acid by Strong Base 14 12 10 8 pH (0.1 M acetic) 6 4 2 0 0 0.05 0.1 0.15 Volume of Base 0.2 0.25 Strong/Strong Titration Curve V base 0 50 90 95 99 99.9 100 V total 100 150 190 195 199 199.9 200 [H+] 1M .5/1.5 .1/1.9 .05/1.95 .01/1.99 .001/1.999 0/2 pH 0 0.48 1.28 1.59 2.30 3.30 7.00 Acid-Base Indicators Indicators: molecules whose acid-base conjugates have distinct colors. Color change occurs as acid/base ratio nears 1, i.e., as pHpKa (of indicator!) Extreme sensitivity of pH to titrant volume near endpoint makes use of indicators quantitative. Match pKindicator to pH at equivalence. pH at Equivalence Sample is gone, replaced by conjugate at original number of moles. [conjugate]0 = [sample]0 (V0 / Vtotal) F [conjugate]equilibrium = F – x (back rxn with water) Kconjugate = x2 / (F – x) or x [FKc]½ pHequivalence = px or 14 – px = 8.72 (acetic) pKindicator pHequivalence is [Ind–]/[Ind]1. pH in the Buffer Region Ka = [H+] [A –] / [HA] = [H+] [S ]/[HA] log Ka = log[H+] + log( [S]/[HA] ) pKa = pH – log( [S]/[HA] ) pH = pKa + log( [S]/[HA] ) neither S nor HA=0 Henderson-Hasselbalch Equation pOH = pKb + log( [BH +]/[B] ) Concentration ratios = mole ratios! Solubility Product AxBy(s) x Ay+(aq) + y Bx–(aq) Q = [Ay+]x [Bx–]y for arbitrary concentrations K = [Ay+]eqx [Bx–]eqy for saturation conc. Q < K implies no solid Q = K implies saturated solution Q > K super saturation difficult to achieve! Spontaneously precipitates. Calculating Solubility Product Make a saturated solution. Remove it from its precipitate. Evaporate to dryness and weigh solid. Convert to moles n of solid in original V. If AxBy then [Ay+]=x(n/V) ; [Bx–]=y(n/V) Ksp = (xn/V)x (yn/v)y x and y have enormous influence Solubility and pH If dissolved ions are conjugates of weak acid, say, both Ksp and Kb must be satisfied. Ksp fixes [A–] at equilibrium value, and Kb establishes [OH–] and [HA], for example. If Ka–1 and [H+] can lower [A–] below the solubility limit, acid can dissolve the solid. (Assuming solid is limiting reactant.) Dissolving Oxides Ag2O + H2O 2 Ag+ + 2 OH– (410–16) 2 H+ + 2 OH– 2 H2O (10+14)2 Ag2O + 2H+ 2Ag+ + H2O (410+12) Equilibrium lies far to right for modest acid. Cu2O + H2O 2 Cu+ + 2 OH– (410–30) Cu2O + 2H+ 2Cu+ + H2O (410–2) Only concentrated acids will suffice. Complex Equilibria Empty or unfilled metal d-orbitals are targets for lone pair electrons in dative or coordinate-covalent bonding. Square planar or octahedral (and beyond) geometries of ligands (e– pair donors) bind to metal atoms to make complexes. Ligands can be neutral (H2O, NH3, CO … ) or charged (Cl–, CN–, S2O32– … ). Complex Equilibrium Constant Exchange of ligands (labile) is governed by equilibrium constants. Solid solubilities are thus influenced by ligand availability. H2O always available (aq), but it’s not the strongest ligand. Serial replacement of H2O by other ligands leads to a sequence of equilibrium constants. vs. K Polyprotic acid constants proceed proton by proton: HSO4–(aq) H+(aq) + SO42–(aq) Ka2=10–2 Ligand addition constants, , are cumulative instead: Ag+(aq) + 2 I–(aq) AgI2–(aq) 2=1011 really Ag(H2O)4+ + 2 I– Ag(H2O)2I2– + 2 H2O