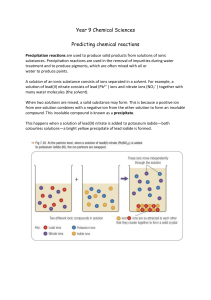
For each solution, determine the soluble ions.
advertisement

For each solution, determine the soluble ions. A. B. C. D. Potassium Iodide (aq) Barium Nitrate (aq) Lead Nitrate (aq) Sodium Sulfate (aq) Mix the following solutions in pairs A. B. C. D. Potassium Iodide Barium Nitrate Lead Nitrate Sodium Sulfate • When finished, try Silver Nitrate with Potassium Iodide. Determine the precipitates. Solubility of Ions Always Sometimes K+ IPb2+ NO3Ag+ Ba2+ SO42Na+ Precipitate Reactions • Precipitates • Solubility rules • Using the rules of solubility Reaction of Pb(NO3)2 and KI • Lead II Nitrate and Potassium Iodide • Both are strong electrolytes • That means they completely dissociate into ions • Adding the solutions together will create something new… • A chemical change the evidence is the precipitate. Look closer at Lead (II) Nitrate Strong Electrolyte Ions completely dissociate into: Lead Pb2+ ions Nitrate NO3 - Ions Pb (NO3)2 The KI does the same thing • Posassium K+ ions and Iodide I- ions form. They are floating around in the water. (an aqueous solution) When the lead and the iodide ions come into contact, they form a precipitate • The precipitate falls to the bottom, spectator ions are left in solution Solubility Rules • All sodium, potassium, ammonium, and nitrate • Rationale: salts are soluble in water. Memorization of solubility rules does • ✘✘Memorization of other “solubility rules” is beyond not deepen the scope of this course and understanding the of the AP Exam Stoichiometry in Aqueous Reactions What volume of 0.200 M copper (II) sulfate is required to react with 50.0mL if 0.100 M NaOH? 1) Write the net ionic equation What volume of 0.200 M copper (II) sulfate is required to react with 50.0mL if 0.100 M NaOH? find the moles of each reactant needed: nOH- = nCu2+ =