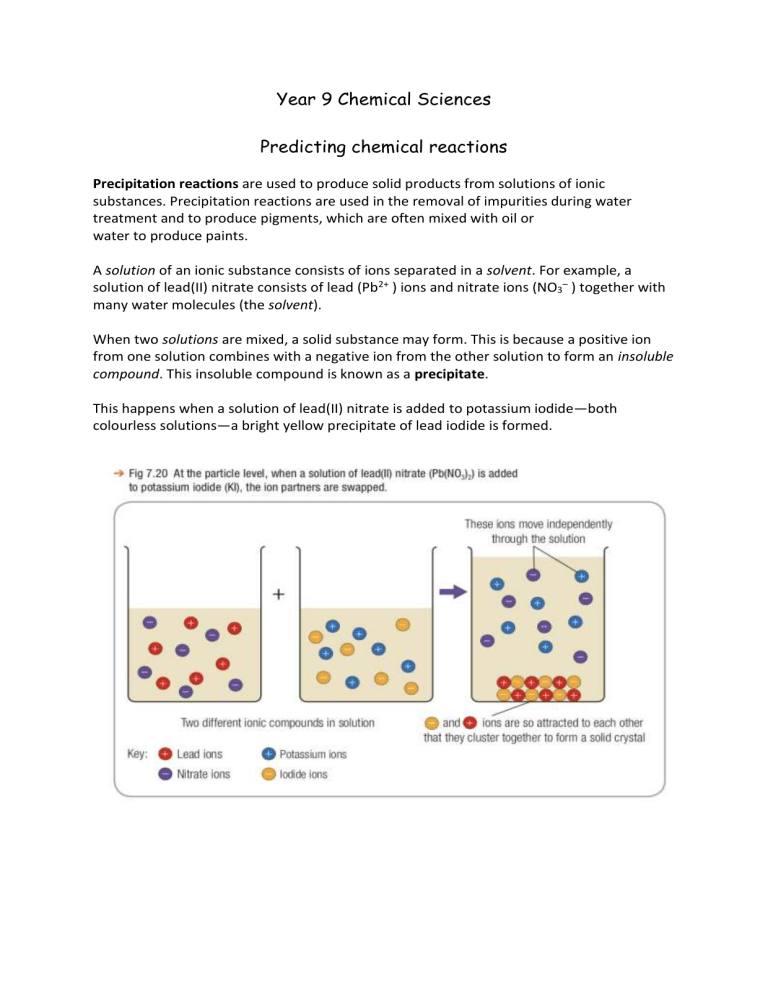
Year 9 Chemical Sciences Predicting chemical reactions Precipitation reactions are used to produce solid products from solutions of ionic substances. Precipitation reactions are used in the removal of impurities during water treatment and to produce pigments, which are often mixed with oil or water to produce paints. A solution of an ionic substance consists of ions separated in a solvent. For example, a solution of lead(II) nitrate consists of lead (Pb2+ ) ions and nitrate ions (NO3– ) together with many water molecules (the solvent). When two solutions are mixed, a solid substance may form. This is because a positive ion from one solution combines with a negative ion from the other solution to form an insoluble compound. This insoluble compound is known as a precipitate. This happens when a solution of lead(II) nitrate is added to potassium iodide—both colourless solutions—a bright yellow precipitate of lead iodide is formed. Determining molecular formula using the 3 step Swap and Drop method Step 1 Identify Ions Step 2 Swap and Drop (circle numbers drop them down) Step 3 Write the final formula (lose the 1’s) Example: Lead (ii) Nitrate Pb 2+ NO3 1Pb 1 (NO3) 2 Pb(NO3)2 When the following ionic compounds are added together a coloured precipitate forms. Your task is to predict the chemical equation that represents each precipitation reaction. Your equation must include a correct molecular formula for each ofl the reactants and products. Iron (II) Nitrate and Potassium Iodide Magnesium Chloride and Sodium Hydroxide Potassium Chromate and Copper Sulfate Remember to follow the 4 step procedure shown in class Step 1 Determine molecular formulae of reactants Step 2 Predict the products that form after the precipitation reaction (write a draft chemical equation) Step 3 Determine molecular formulae of products Step 4 Write the final chemical equation




