ALKYnes sem 1: 2011/2012
advertisement

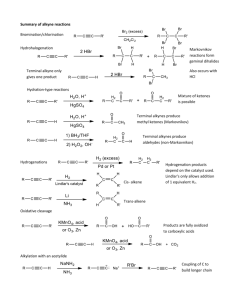
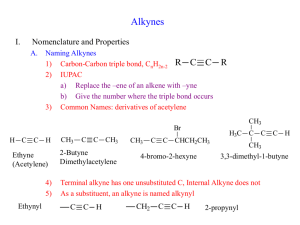
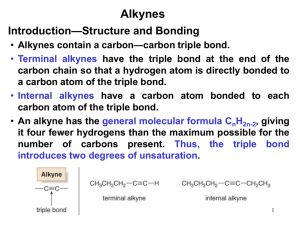
Khadijah Hanim bt Abdul Rahman School of Bioprocess Engineering, UniMAP Week 5: 13/10/2011 khadijahhanim@unimap.edu.my Structure, - nomenclature and naming alkynes DEFINE and ILLUSTRATE the principle in naming alkynes on few examples Reaction of alkynes - EXPLAIN and DISCUSS the reactivity of alkynes in electrophilic addition reaction. Alkyne is a hydrocarbon that contains a carboncarbon triple bond. Alkyne has 4 fewer hydrogens than an alkane. The general molecular formula for an acyclic alkyne is CnH2n-2 and for cyclic alkyne is CnH2n-4. Only - a few alkynes are found in nature. capillin- fungicidal activity Ichthyothereol- convulsant used by Amazon people for poisoned arrowheads Enediynes- antibiotic/anticancer properties Few drugs on the market that contain alkyne functional groups- not naturally occuring Synthesized Replacing ‘ane’ ending of alkane name with ‘yne’. The longest continuous chain with functional group is no in the direction that gives the alkyne functional group suffix as low no as possible. Terminal alkynes- triple bond is at the end of the chain Internal alkynes- triple bonds located elsewhere along the chain Common nomenclature-alkynes are named as substituted acetylenes. Common name is obtained by stating the names of alkyl groups in alphabetical order. If counting from either direction leads to the same no for the functional group suffix, correct systematic name- the one that contains the lowest substituent no. If substituent- more than 1 substituents- listed in alphabetical order Draw - - the structure for the following: 1-chloro-3-hexyne Cyclooctyne Isopropylacetylene 4,4-dimethyl-1-pentyne Find the longest continuous chain containing both functional groups Put both suffixes at the end of the name The ‘ene’ ending should be first with terminal e omitted. The no indicating the location of the firststated functional group- placed before the name of the parent chain. No indicating the location of 2nd-stated functional group is placed immediately before the suffix for that functional group. If the 2 functional groups are a double bond and triple bond, chain is numbered to produce a name containing the lower no., regardless of which functional group gets the lower no. If the same low no is obtained in both directions, the chain is numbered in the direction that gives the double bond the lower no. If the 2nd functional group suffix has a higher priority than the alkene suffix, the chain is numbered in the direction that assigns the lower no to functional group with higherpriority suffix. The highest priority functional group is assumed to be at the 1-position in cyclic compounds. Give the systematic name for each of the following: Physical properties similar to alkanes Insoluble in water and soluble in nonpolar solvents such as benzene or diethyl ether Less dense than water Boiling points that increase with increasing molecular weight Alkynes are more linear than alkenes- triple bond is more polarizable than a double bond These 2 features- stronger van der waals interactions Alkyne has higher boiling point than an alkene with the same no of carbons. Internal alkenes have higher boiling points than terminal alkenes. Structure of ethyne: each carbon is sp hybridized, each has 2 sp orbitals and 2 p orbitals. 1 sp orbital overlaps the s orbital of a hydrogen, and the other overlaps an sp orbital of the other carbon. Because the sp orbitals are oriented as far from each other as possible to minimize electron repulsion, ethyne is a linear molecule with bond angle 180o. The triple bond is formed by each of the 2 p orbitals on 1 sp carbon overlapping the parallel p orbital on the other sp carbon to form 2 π bonds. The electrostatic potential maps for 2butyne show that it look like a cylinder of electrons wrapped around the σ bond. A triple bond is composed of a s bond and two p bonds Carbon-carbon triple bond is shorter and stronger than carbon-carbon double bond A π bond is weaker than σ bond. The relatively weak π bond allow alkynes to react easily. Alkyl groups stabilize alkynes by hyperconjugation. Therefore, internal alkynes are more stable than terminal alkynes Thus, the alkyl groups stabilize alkenes, alkynes and carbocation. Alkyne is an electron-rich molecule- nucleophile React with electrophile Mechanism for electrophilic addition reaction: Z-Stereoisomer The relatively weak π bond breaks because the π electronattracted to the electrophilic proton The positively charged carbocation intermediate reacts rapidly with the negatively charged chloride ion Alkynes- electrophilic addition reactions Like alkenes- electrophilic addition to a terminal alkene is regioselective. In addition, the addition reaction of alkynes have a feature that alkenes do not have: because the product of the addition of an electrophilic reagent to an alkyne is alkene, a 2nd electrophilic addition reaction may occur if excess hydrogen halide is present. Alkynes less reactive in electrophilic addition reactions Reactivity depends on ∆G++- which depends on the stability of reactant and the stability of the transition state For alkyne to be less stable and less reactive than alkene, 2 conditions must hold: - The transition state for rate-limiting step of an electrophilic addition reaction for an alkyne must be less stable than the transition state for the first step of electrophilic reaction for an alkene and - The difference in the stabilities of the transition states must be greater than the difference of the reactants, so that ∆Galkyne >∆Galkene Why is the transition state for the 1st step of an electrophilic addition reaction for an alkyne less stable than that for an alkene? - Hammond predicts- the structure of transition state for the 1st step reaction resemble the structure of carbocation intermediate-product of 1st step. -Carbocation formed when a proton adds to an alkyne- vinylic cation. Whereas, the carbocation formed when proton adds to an alkene- alkyl cation. - A vinylic cation has +ve charge on vinylic carbon,which is more electronegative than the sp2 carbon of alkyl cation- less stable than a similarly substituted alkyl cation. A vinyl cation has +ve charge on an sp carbon- more electronegative than sp2 carbon of an alkyl cation- less able to bear a positive charge Hyperconjugation-less effective in stabilizing a charge on a vinylic cation than on an alkyl cation.