Document
advertisement

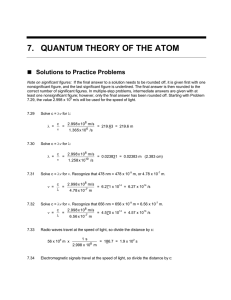
Bohr’s Shell Model EXCITED state e–’s absorb (+) energy, move to outer levels (n=2 to n=5) 5 2 e–’s emit (–) energy, move back to inner levels (n=5 to n=2) GROUND state ∆E quantum: amount of energy absorbed or emitted as electrons transition fixed energy levels. Electron Energy and Light The distance between same points on wavelength () (m) adjacent waves is the _______________. The number of waves passing a point per frequency () (Hz)(s–1) unit of time is the ______________. If both waves move at the same speed, and are inversely proportional which has more energy? R O Y G B I V Electromagnetic Spectrum Lowest Energy Highest Energy (higher ) (shorter ) All EM radiation travels at the same speed: the speed of light (c), 3.00 108 m/s. joules (J) c = (given on test) E = h (c) speed of light (h) Planck’s constant = 3.00 x 108 m/s = 6.63 x 10–34 shorter higher E higher , _______ higher , ______ (inversely) (directly) Calculate energy (E) from frequency () or wavelength (): ↔ ↔E Sample Calculation 1: c = λ Calculate the wavelength of the yellow light emitted by a sodium lamp if frequency of the radiation is 5.10 x 1014 Hz. (3.00 x 108) = λ (5.10 x 1014) (3.00 x 108) = λ (5.10 x 1014) λ = 5.88 x 10–7 m Sample Calculation 2: c = λ Calculate the frequency of blue light with a wavelength of 6.05 x 10–7 m (or 605 nm). (3.00 x 108) = (6.05 x 10–7) (3.00 x 108) = (6.05 x 10–7) = 4.96 x 1014 s–1 Sample Calculation 3: E = h Calculate the energy of the yellow light emitted by a sodium lamp if frequency of the radiation is 5.10 x 1014 Hz. E = (6.63 x 10–34) (5.10 x 1014) E = 3.38 x 10–19 J Sample Calculation 4: c = λ E = h Calculate the energy of blue light with a wavelength () of 6.05 x 10–7 m (or 605 nm). c = λ (3.00 x 108) = (6.05 x 10–7) (3.00 x 108) = (6.05 x 10–7) = 4.96 x 1014 s–1 E = h E = (6.63 x 10–34) (4.96 x 1014) E = 3.29 x 10–19 J Quick Quiz! 1. Which of the following relationships is true? A. Higher-energy light has a higher frequency than lower-energy light. B. Higher-energy light has a longer wavelength than lower-energy light. C. Higher-energy light travels at a faster speed than lower-energy light. D. Higher-frequency light travels at a slower speed than lower-energy light does. Quick Quiz. 2. The energy of EM radiation is greatest for… A. visible light. B. ultraviolet light. C. infrared light. D. X-ray radiation. Quick Quiz. 3. The longer the wavelength of light, the… A. higher the frequency. B. higher the energy. C. lower the frequency. D. lower the energy.