Energy & Water
advertisement

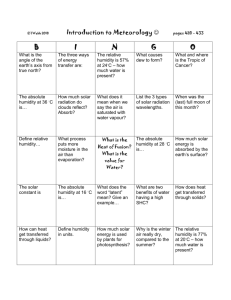
Energy & Water Weather Dynamics Science 10 Planet Water • Earth’s surface is only 30% land (70% water) and Clouds cover much of this land. • For this reason most of the incoming solar radiation that penetrates the atmosphere strikes water. • Due to this, the interactions between solar energy and water have a major influence on weather. • Is it any wonder that people have said that “Planet Earth” should be named “Planet Water”? Interactions • Earth interacts with solar radiation in two ways: • Absorbing the radiation and heating the Earth OR • Reflecting the radiation back out into space and having no direct effect on us and our planet. Interactions • The type of interaction that occurs between solar radiation and water depends on the state of the water: • Fresh, white snow reflects up to 90% of incoming radiation • Ice reflects about 50% of incoming radiation • Water reflects only 7% of this radiation (So water absorbs 93% of the incoming solar radiation. Interactions • For this reason, if Earth was covered entirely with water, after 25 years the water would have absorbed enough solar radiation to boil the world’s oceans and make life on Earth impossible. Water is the only substance on Earth that occurs in nature in all three states – solid, liquid, and gas. • This is a scary thought, however Earth’s temperature has remained fairly constant for thousands of years. Why do you think this is? Interactions • The temperature of oceans and very large lakes are relatively constant because: • Water has some very unique properties. Dun Dun Dun! How do you catch a unique bird? You gotta unique up on it! How do you catch a tame bird? Tame way! Unique up on it! Water Properties: Property 1 • Water has a large specific heat capacity. • Specific heat capacity is defined as the amount of heat that is required to raise the temperature of one gram of a substance one degree Celsius. • Specific heat capacity is measured in J/g∙˚C • Scientists have measured specific heat capacities (c) for many substances. Specific Heat Capacities Substance in Nature Pure water Specific Heat Capacity (J/g∙˚C) Sea water 3.89 Dry air 1.00 Wet mud 2.51 Brick 0.84 Granite 0.79 Limestone 0.92 4.18 Specific Heat Capacities Liquid Specific Heat Capacity (J/g∙˚C) Water 4.18 Methanol 2.55 Ethanol 2.46 Hexane 2.26 Toluene 1.80 Specific Heat Capacity • The amount of heat required to heat 1 gram of a substance 1 degree can be calculated with a formula: Q=mc∆T Amount of heat = Q Mass/amount of substance = m Specific heat capacity = c Change in temperature = ∆T ∆ means “change in” Problem: Compare the increase in the temperature of 1.0 g of water and 1.0 g of ethanol when they each absorb 10J of thermal energy. Ethanol: Amount of heat absorbed (Q) = 10J Mass of ethanol (m) = 1.0g SHC of ethanol (c) = 2.46 J/g∙˚C Q=mc∆T 10J = (1.0g)( 2.46 J )∆T g C 10 J = ∆T 2.46 J / C 4.1˚C = ∆T Problem: Compare the increase in the temperature of 1.0 g of water and 1.0 g of ethanol when they each absorb 10J of thermal energy. Water: Amount of heat absorbed (Q) = 10J Mass of ethanol (m) = 1.0g SHC of ethanol (c) = 4.18 J/g∙˚C Q=mc∆T 10J = (1.0g)( 4.18 J )∆T g C 10 J = ∆T 4.18 J / C 2.4˚C = ∆T Problem: Compare the increase in the temperature of 1.0 g of water and 1.0 g of ethanol when they each absorb 10J of thermal energy. • When 1.0g of water absorbs 10J of energy, the temperature increases by 2.4˚C, • But when 1.0g of ethanol absorbs 10J of energy, the temperature increases by 4.1˚C (almost double). • This is because water has a higher specific heat capacity! • What does this mean? We can attribute more heat to water than most other substances and it will not warm up as fast. Water Properties: Property 2 • Even though water requires more energy than most to heat up, it still absorbs a lot of solar radiation and therefore would, overtime, gradually heat up. • This doesn’t explain why Earth remains at a constant temperature entirely. • A second property of water which helps to explain this is called Heat of Vaporization. Heat of Vaporization • Heat of vaporization is the amount of heat required to change 1 gram of a substance from a liquid to a gas. • If the gas condenses back into a liquid, the same amount of energy is released as heat. • When water absorbs solar energy, some of this energy is used to evaporate water and becomes unavailable to heat up our oceans and other large water bodies. • Evaporation keeps us cool ! Heat of Vaporization Liquid Water Heat of Vaporization (J/g) 2260 Methanol 1076 Ethanol 855 Hexane 335 Toluene 363 Heat of Vaporization • As you can see, water uses a large amount of its absorbed energy in evaporation, giving water one more tool in allowing Earth to keep its constant temperature • Water has a HIGH heat of vaporization. • The amount of energy (heat) required to evaporate a substance can also be calculated using the formula: Q=m∆H˚vap Amount of Energy = Q Mass of substance = m Heat of vaporization = ∆H˚vap Problem: A large hole in your driveway collects 2.7kg of water. How much energy is required to evaporate all of the water? • Mass of water = 2.7kg • Heat of vaporization of water = 2260J/g 2.7kg to g 1kg = 1000g Q=m∆H˚vap Q = (2700g)(2260J/g) Q = 6 102 000 J = 2700g One joule in everyday life is approximately: • the energy required to lift a small apple one metre straight up. • the energy released when that same apple falls one metre to the ground. • the energy released as heat by a person at rest, every hundredth of a second. Water Properties: Property 3 • Although our two previous properties are giving us some ideas as to how the Earth remains at a constant temperature, we still haven’t received the entire picture. • A third unique property of water is its large Heat of Fusion Heat of Fusion Very similar to our Heat of Vaporization, • Heat of fusion is the amount of heat that is required to melt 1.0g of a solid into a liquid. • It is also the amount of energy released by a liquid when 1.0g of liquid freezes to a solid. • Water has a large heat of fusion as well, using some of the energy it absorbs in order to turn ice into liquid water. Heat of Fusion Substance Water Heat of Fusion (J/g) 333 Methanol 100 Ethanol 109 Hexane 152 Toluene 72 Heat of Fusion • Water’s high heat of fusion and vaporization together help to use up absorbed solar radiation preventing our oceans from boiling • Heat of fusion can also be calculated by the formula: Q=m∆H˚fus Amount of Energy = Q Mass of substance = m Heat of fusion = ∆H˚fus Problem: A snowplough has pushed 750kg of snow into a huge pile at the school. How much energy is required to melt the pile? •Mass of water (solid) = 750kg •Heat of fusion of water = 333J/g 750kg to g 1kg = 1000g = 750 000g Q=m∆H˚fus Q = (750 000g)(333J/g) Q = 249 750 000 J Water in the Air • When there is as much water vapour in the air as possible it is called saturated. • If water cools, or if more water evaporates, some of the water vapour in the air will condense into water droplets. Humidity • Humidity is the amount of water vapour in the air • Absolute humidity is the actual amount of water vapour in the air, in grams. • Relative humidity, which you would hear on a weather network or news broadcast is the percentage of water vapour in the air. • Relative humidity would be calculated by taking the amount of water vapour in the air (Absolute humidity) and dividing it by the saturated amount (maximum amount of water that can exist at that temperature). The Water Cycle • Evaporation and Condensation are occurring throughout the world, at all times. • About 23% of all incoming solar radiation causes liquid water to evaporate into water vapour. • Most evaporation comes from oceans, although other water bodies contribute. • Another important source of evaporation are plants. Plants draw water up from their roots and the water evaporates out through their leaves. The Water Cycle • Due to the constant warming and cooling of water at different places on Earth, much of the solar radiation that would boil this planet to a crisp is used up in order to help sustain an environment where temperature is able to remain fairly constant. • Even putting on your coat and going outside in the winter causes this exchange of warm and cold air. Do You Understand? 1. 2. 3. 4. 5. 6. 7. 8. How does the specific heat capacity of water influence the temperature of a large lake? Compare heat of vaporization with heat of fusion. What type of energy change occurs when liquid water freezes? What happens when saturated air cools? Explain what a weather reporter means by “ The relative humidity is 62 percent.” If steam condense on skin, it can cause a very serious burn. Use the concept of heat of vaporization to explain the cause of the burn. Assume that a small fish pond contains 4000kg of water. If it absorbs 6.15 x 10 J of solar energy in one day, and none is lost to evaporation, how much will the temperature of the water rise? If the same amount of energy was used to evaporate water, how much water would evaporate?