Review1
advertisement

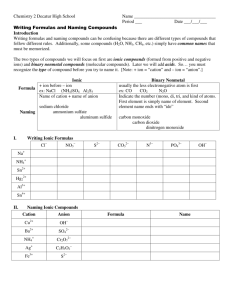
REVIEW CHAPTERS 1, 2, 3 Chemistry Matter and Measurement 1 Chapter Overview 1.1 Chemical Foundations 1.2 Scientific Method :Observation – hypothesis – law – theory - experiment 1.3-1.7 Classification of Matter A. Physical property Observable without changing the identity B. Chemical Property Observable only by changing the identityChemical reactions 1) 2) Physical Change Chemical Change 1 Units of Measurements (table 1.1 and 1.2) SI units: length, mass, time, temperature, amt of substance meter, kilogram, second, Kelvin , mole Table 1.2 2 Uncertainity in Measurements Precision and Accuracy, standard deviation and % error 3 Significant Figures and Calculation- exponential notation • Significant figures •Significant Figures in Calculations -Multiplication and Division: Answer with lowest Sig. Fig -Add/Subtract: Answer with least decimal place -All together- Do parentheses first, then add/subtract-with decimal places, then multiply-Divide minimum sig fig. • Significant Figures: Writing Numbers to Reflect Position A. How many digits can I report? How many should I report? B. Certain digits and estimated digits C. Counting significant figures 1. All nonzero digits are significant 1234 = 4 Sig fig 2. Interior zeros are significant 505 = 3 sig fig 3. Trailing zeros after a decimal are significant 55.00 = 4 sig fig 4. Leading zeros are not significant 0.012 = 2 sig fig 5. Zeros at the end of a number, without a decimal point, are ambiguous 150 = ambiguous D. Exact numbers 4 Converting from One Unit to Another (UNIT 1 to UNIT 2) A. Units are important, most numbers get one B. Include units in all calculations C. Conversion factors (Unit you have comes in the bottom, unit you want comes in the TOP) Unit 1 X Unit 2 = UNIT 2 Unit 1 D. Significant figure of the final answer depends on UNIT 1 given in problem NOT the sig. fig of the conversion factor(s) Temperature: Random Molecular and Atomic Motion Conversions F - 32 C 1.8 And 1.8 C 32 F K C 273 1.8 Density (D= Mass/Volume) A. Mass per unit volume (D= Mass/Volume) B. Derived unit Volume = Mass/Density Mass = Density X Volume C. Can be used as a conversion factor between mass and volume Atoms, Molecules and Ions 2/3 • 2.1 and 2.2 Laws of Chemical Combination, Chemical Laws • 2.3 John Dalton and the Atomic Theory of Matter • 2.1 Divisible Atoms (Protons(+1, 1u), electrons(-1, 1/2000th u), neutrons(0,1u)) Isotopes – atoms of same element having different mass no. (due to DIFFERENT # of neutrons) 2.2-2.4 Atomic Masses Calculation problems ( Isotopes and abundances) Isotope symbols for atoms and ions 2.5 The Periodic Table: Elements Organized Looking for Patterns: The Periodic Law and the Periodic Table A. Mendeleev (1834 - 1907) B. Periodic law C. Metals D. Nonmetals E. Metalloids, also known as semiconductors F. Individual group names 1. Group 1 – alkali metals 2. Group 2 – alkali earth metals 3. Group 7 – halogens 4. Group 8 – noble gases 2.6 Molecules and Ions •Nomenclature (between 2 or more non metals!!!) •Use of prefix and suffix (di, tri etc) •Prefix)name of first element + (prefix) base name of second element + ide •More positive non metal written first 2.7 Ions and Ionic Compounds Naming Ionic Compounds A. Type I compounds 1. Metal in compounds forms only one type of ion 2. Most main group metals form type I compounds B. Naming type I binary ionic compounds 1. Name of cation (metal) + (base name of anion + ide) 2. Example, NaCl is sodium chloride C. Type II compounds 1. Metal forms more than one type of ion 2. Transition metals usually, but not exclusively, form type II compounds D. Naming type II binary ionic compounds 1. Name of cation + (charge of cation) + (base name of anion + ide) 2. Charge of cation given in roman numerals 3. FeCl3 is iron (III) chloride E. Naming ionic compounds containing a polyatomic ion 1. Use the same procedure as ionic compounds 2. Use name of polyatomic ion, not constituent atoms 3. Example, NaNO3 is sodium nitrate 3. 6 Acids, Bases, and Salts (Nomenclature of acids) Naming Acids A. Molecular compounds that dissolve in water to form H+ ions B. Binary acids 1. Hydrogen and nonmetal 2. (Hydro + base name of nonmetal + ic) + acid 3. Example, HCl is Hydrochloric acid C. Oxyacids 1. Hydrogen and polyatomic oxyanion 2. Oxyanions ending with –ate a. (Base name of oxyanion + ic) + acid b. Example, HNO3 is nitric acid 3. Oxyanions ending with –ite a. (Base name of oxyanion + ous) + acid b. Example, HNO2 is nitrous acid Combined concepts from Chapter 2 and 3 2.9 2.10 3 3.1 3.2 Counting by weight, The Mole and Molar Mass Molecular Masses and Formula Masses, Percent Composition Emprical/Chemical Formulas from Mass Percent Composition Chemical Equations Writing and Balancing Chemical Equations Chapter 3 Some Electrical Properties of Aqueous Solutions Reactions Involving Oxidation and Reduction-Redox, Single Dispalcement Reactions -Oxidation involves LOSS of Electrons “OIL” -Reduction involves GAIN of Electrons “RIG” Oxidation Number (ON) rules: 1. 2. 3. 4. 5. 6. Sum of ON 0 for a compound; sum of ON ionic charge for an ion. 1A elements in compounds have ON 1 ; 2A elements in compounds have ON 2 . In compounds F has ON 1 . In compounds H has ON 1 . In compounds O has ON 2 . In binary compounds, 7A elements have ON = –1; 6A elements have ON = –2; and 5A elements have ON = –3 Single -displacement reaction,. Activity series in metals Look up Single-Double displacement lab for Practice EXAM 1- 100 POINTS 10 points Bonus question!! Part 1 Multiple Choice –Show calculations for partial/full credit 15 questions Part 2 Density, Moles, atomic mass, empirical formulas, yield, limiting agent questions, Single-Double displacement reactions Bonus question: