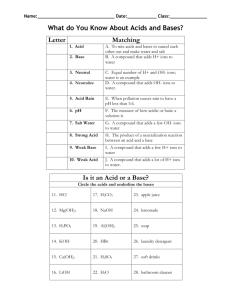
ACID (A) or BASE
advertisement
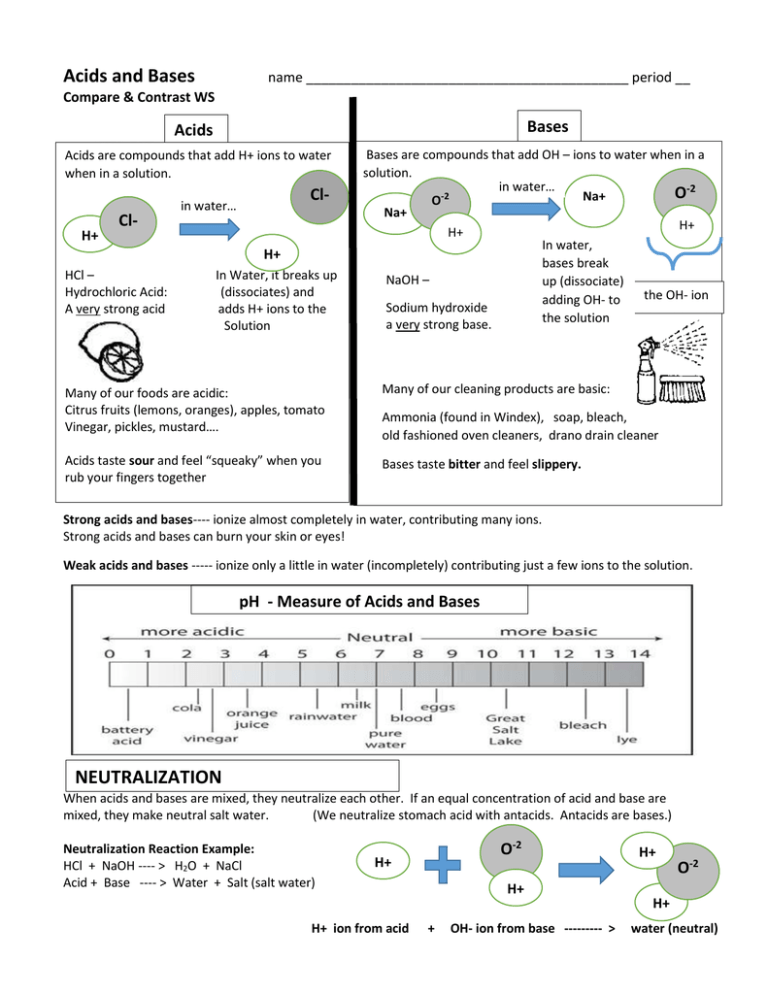
Acids and Bases name ___________________________________________ period __ Compare & Contrast WS Bases Acids Acids are compounds that add H+ ions to water when in a solution. H+ Cl- Cl- in water… H+ HCl – Hydrochloric Acid: A very strong acid In Water, it breaks up (dissociates) and adds H+ ions to the Solution Bases are compounds that add OH – ions to water when in a solution. in water… O-2 Na+ O-2 Na+ H+ H+ In water, bases break NaOH – up (dissociate) the OH- ion adding OH- to Sodium hydroxide the solution a very strong base. Many of our foods are acidic: Citrus fruits (lemons, oranges), apples, tomato Vinegar, pickles, mustard…. Many of our cleaning products are basic: Acids taste sour and feel “squeaky” when you rub your fingers together Bases taste bitter and feel slippery. Ammonia (found in Windex), soap, bleach, old fashioned oven cleaners, drano drain cleaner Strong acids and bases---- ionize almost completely in water, contributing many ions. Strong acids and bases can burn your skin or eyes! Weak acids and bases ----- ionize only a little in water (incompletely) contributing just a few ions to the solution. pH - Measure of Acids and Bases NEUTRALIZATION When acids and bases are mixed, they neutralize each other. If an equal concentration of acid and base are mixed, they make neutral salt water. (We neutralize stomach acid with antacids. Antacids are bases.) Neutralization Reaction Example: HCl + NaOH ---- > H2O + NaCl Acid + Base ---- > Water + Salt (salt water) O-2 H+ H+ ion from acid H+ + OH- ion from base --------- > H+ O-2 H+ water (neutral) pH and Acid Rain Plants and animals need water close to neutral (pH 7 ) to survive. Due to pollution from combustion reactions, rain today can be acidic. Rain less than pH 5.6 is called acid rain. Acid rain can kill plants, kill fish, cause asthma, and other physical problems. Acid rain also eats away pavement, brick, rock, statues, and historical landmarks. The Roman ruins , the pyramids of Egypt and other treasures of the world are being slowly dissolved away by acid rain. More damage has been done in the last century than in the last 2000 years. Without stopping pollution (and acid rain) these treasures may be lost forever. ____ 1. Acid A. To mix acids and bases; to cancel each other out; to make salt water. ____ 2. Base ____ 3. Neutral B. A compound that adds H+ and OH – ions to water. ___ 4. Neutralize C. Equal number of H+ and OH – ions; water is an example. ____5. Acid Rain D. A compound that adds OH- ions to water. ____ 6. pH ____ 7. Salt Water ____ 8. Strong Acid ____ 9. Weak Base ____10. Weak acid E. When pollution causes rain to be acidic (pH of less than 5.6 ) A. The measure of acids and bases B. A compound that adds a few OH- ions to water C. The product of a neutralization reaction between acid & base. D. A compound that adds a few H+ ions to water. E. A compound that adds a lot of H+ ions to water. Circle the acids and underline the bases. HCl H2CO3 H3PO4 H2SO4 NaOH Mg(OH)2 Ca(OH)2 HNO3 KOH LiOH HF Solution A (pH 4 ) Solution B (pH 2) Which one has more H+ ions?__________ Which one has less OH- ions?___________ ACID (A) or BASE (B) ? Has fewer OH- ions: _____ pH of 1 to 7 : _____ Solution C (pH 11 ) Has more H+ ions : _____ pH of 7 to 14 : _____ Which one has more OH- ions?________ Has fewer H+ ions: _____ feels slippery : _____ Which one has less H+ ions?__________ Has more OH – ions: _____ tastes sour: _____ Circle names that WILL use “hydro” in the naming the acid: HBr H2SO3 HF Circle the bases that DO need a roman numeral H3C2O4 in the name: HClO3 Only 2 symbols HI Solution D (pH 2) HC2O3H2 more than 2 symbols LiOH CuOH Fe(OH)3 Ca(OH)2 Sr(OH)2 Complete and balance this neutralization equation: ____ KOH + ______ H2SO4 -------- > ______________ + ______________ Al(OH)3 Cr(OH)2