Testing PH - Cloudfront.net
advertisement

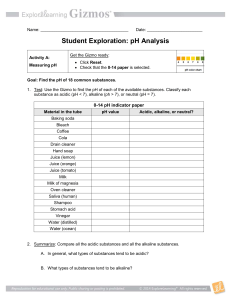
Testing pH This is the title part Introduction Background: Universal indicator paper changes color depending on the pH of the solution. Acidic things have a low pH and lots of Hydrogen. (0-6) Basic things have a high pH and a low amount of Hydrogen. (8-14) Things that are neutral are at 7 pH is a scale of 0-14 pH scale LOTS OF HYDROGEN IONS FEWER HYDROGEN IONS The pH scale measures how acidic or basic a substance is. The pH scale ranges from 0 to 14. A pH of 7 is neutral. A pH less than 7 is acidic. A pH greater than 7 is basic. http://www.youtube.com/wa tch?v=M8tTELZD5Ek Purpose: Which substances that you have do you think will be basic, neutral, and acidic? Hypothesis: I think the: Lemon juice is…because… Milk… is…because… Windex… Ammonia... Water… Vinegar… Materials and Equipment Beakers pH indicator paper See hypothesis for substances Procedure Test each solution with a new pH paper indicator strip. Write down the results for each test. DATA Construct a pH scale ranging from 0-14 Place the solutions on this scale. DATA Solution Water Milk Ammonia Lemon Juice Windex Vinegar pH # Data Analysis 1. 2. 3. What does your data represent (what is the dependent variable What do the high numbers and low numbers represent? What was the independent variable? Error Analysis 1. What was the control? 2. What things did you hold constant 3. Could you have done anything better or did you make any mistakes? 4. What would you do differently next time? Could you try to make anything more constant to be fair? What other factor would you want to test Make a new hypothesis or what do you want to test for next time? Conclusions 1. Were your hypotheses correct? 2. Did any of these substances surprise you? (which ones did you guess wrong on?) 3. What did you find out about cleaning agents? 4. What did you find out about water? 5. What does changing the pH do to enzymes? References Biology textbook: Nowicki, Stephen, Ph.D. Biology. McDougal Little, 2008 (p. 43). PH SCALE ON YOUTUBE